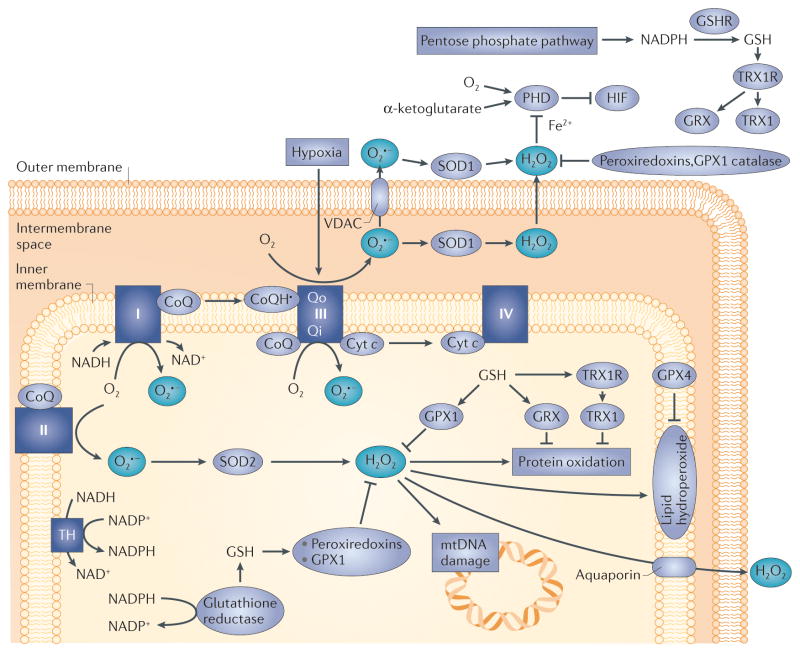
Figure 2. Mitochondrial reactive oxygen species (ROS) generation.
Electrons derived from the oxidation of metabolic intermediates can lead to the generation of ROS at specific sites in mitochondria. Transfer of a single electron to O2 yields superoxide (O2•−) which is converted to hydrogen peroxide (H2O2) by superoxide dismutase in the matrix (SOD2; also known as MnSOD), or in the intermembrane space (SOD1; also known as CuZn–SOD). The H2O2 is degraded in the matrix by glutathione peroxidase 1 (GPX1) or peroxiredoxins (PRDX3 or PRDX5) using reducing equivalents obtained from the oxidation of reduced glutathione (GSH)22–24. Oxidized glutathione (GSSG) is reduced by glutathione reductase, which obtains its equivalents from NADPH oxidation. H2O2 generated in the matrix can oxidize proteins, lipids or mitochondrial DNA (mtDNA). Oxidized proteins are repaired by thioredoxin 2 (TRX2) or glutaredoxin (GRX). TRX2 and GRX are subsequently reduced by thioredoxin reductase 2 or by glutathione. Lipid hydroperoxides are reduced by GPX4. Ultimately, all ROS removal depends on the availability of GSH, which is maintained by the availability of NADPH in the respective compartments. H2O2 can potentially leak to the intermembrane space and the cytosol when excessive ROS generation occurs or antioxidant mechanisms fail. Complexes I, II and III can potentially generate ROS in the matrix compartment. Superoxide can be released to the intermembrane space from complex III, owing to generation from ubisemiquinone at the outer ubiquinone binding site (Qo) of complex III. The electrical gradient across the inner membrane (−180 mV) creates a strong electrical field within the membrane (257 kV per cm) that accelerates superoxide anions from the membrane into the intermembrane space. Paradoxically, cellular hypoxia augments the rate of ROS generation at that site, leading to the production of H2O2 in the intermembrane space13,14,33,36,37. Subsequent diffusion to the cytosol triggers redox-dependent inhibition of prolyl hydroxylases (PHDs), negative regulators of hypoxia-inducible factor-α (HIFα) stabilization. Thus, mitochondria-derived ROS can promote cancer initiation through oxidative stress, and cancer cell progression through the activation of transcription by HIF. CoQ, ubiquinone; cyt c, cytochrome c; GSHR, glutathione reductase; Qi, inner ubiquinone binding site on complex III; TH, mitochondrial trans-hydrogenase; TRX1R, TRX1 receptor; VDAC, voltage-dependent anion channel.