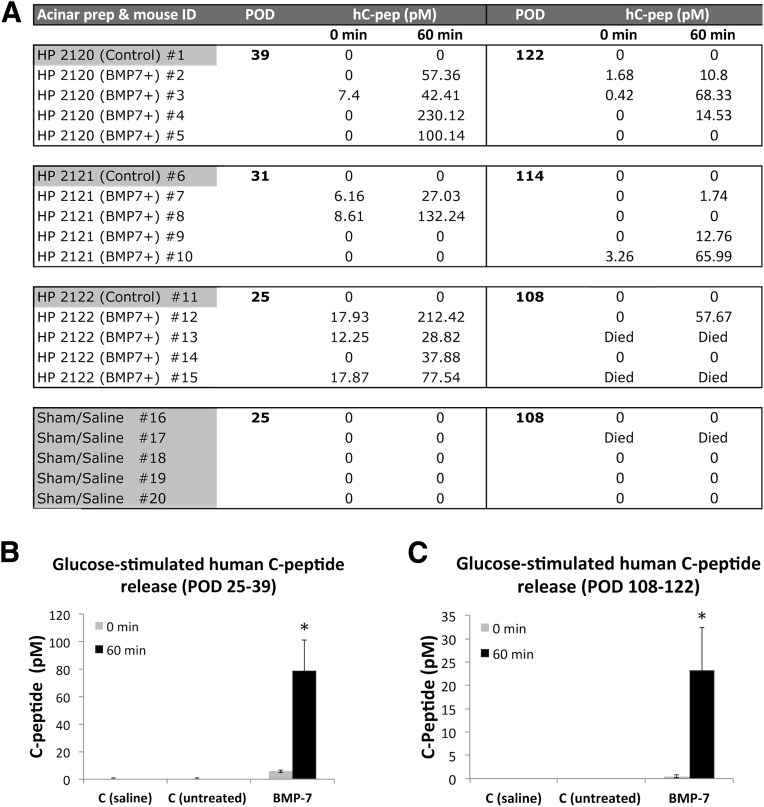
Figure 3.
Functional in vivo characterization of BMP-7–treated hNEPT. A: Human C-peptide determinations in nu/nu, STZ-treated mice transplanted with BMP-7–treated hNEPT, untreated hNEPT, or saline (sham) following the intraperitoneal glucose tolerance test. Left column: hNEPT/mouse recipient identifiers. Second/fifth columns: POD of serum human C-peptide (hC-pep determination). Third/fourth columns: human C-peptide concentrations (pmol/L) obtained prior to (0 min) and 60 min after glucose bolus injection (2.0 g/kg body wt) at PODs 25–39. Sixth/seventh columns: human C-peptide values obtained at PODs 108–122. Average glucose-stimulated human C-peptide release (0 and 60 min, represented by light gray and black columns, respectively) at PODs 25–39 (B) and PODs 108–122 (C). x-axis: C (saline), sham controls; C (untreated), control mice transplanted with untreated hNEPT; BMP-7, mice transplanted with BMP-7–treated NEPT. y-axis: C-peptide (pmol/L). Data are presented as the mean ± SD (n = 12). *P < 0.05. HP, human pancreas.