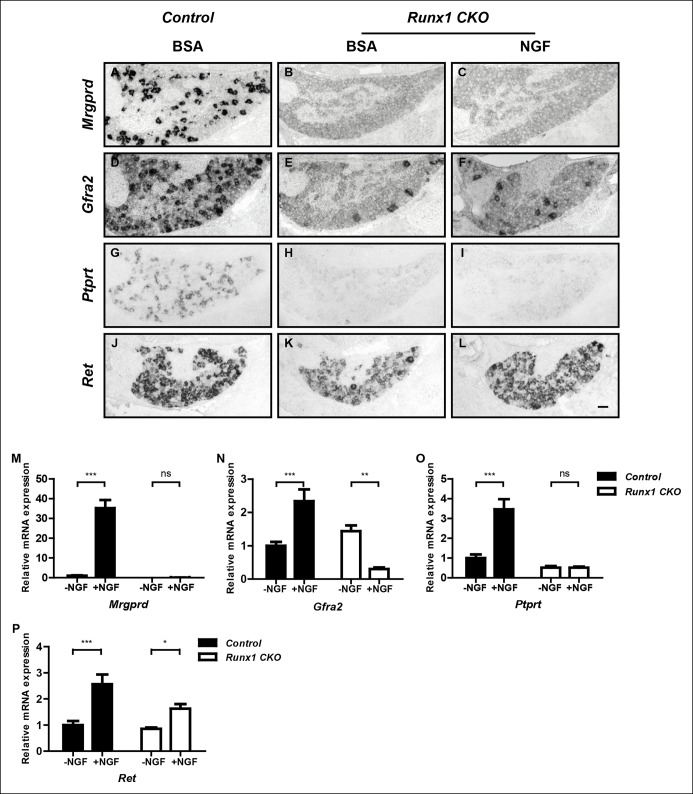
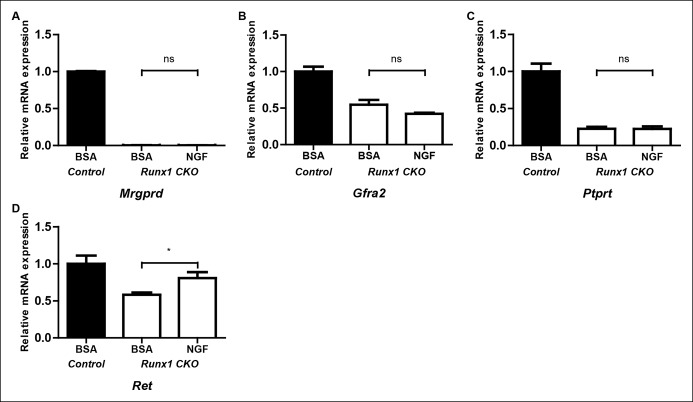
Figure 3. Runx1 functions downstream of NGF to mediate expression of the majority of nonpeptidergic nociceptor-specific genes, whereas it controls Ret expression at least in part by enhancing NGF signaling.
(A–L) In situ hybridization analysis of expression of Mrgprd (A–C), Gfra2 (D–F), Ptprt (G–I) and Ret (J–L) in DRGs of P2 control animals that received BSA injections, Runx1CKO animals that received BSA injections or Runx1 CKO animals that received NGF injections. Note that exogenous NGF administration fails to activate expression of nonpeptidergic-specific genes in Runx1 CKO animals, with the notable exception of Ret, suggesting that Runx1 is a downstream mediator of NGF signaling that is required for expression of the majority of nonpeptidergic-specific genes. The ability of exogenous NGF to upregulate Ret expression in Runx1 CKO animals is most consistent with an indirect role for Runx1 in regulating Ret expression, through enabling NGF signaling. Shown are results representative of at least three independent injection experiments. See also Figure 3—figure supplements 1, 2. (M–P) Real-time PCR analysis of expression of Mrgprd (M), Gfra2 (N), Ptprt (O) and Ret (P) in dissociated DRG neurons from P0 control and Runx1 CKO animals cultured in the presence or absence of NGF. Note that, with the exception of Ret, NGF-dependent expression of these nonpeptidergic-specific genes is completely abolished in the absence of Runx1, further supporting Runx1 as a downstream mediator of NGF in regulating expression of most nonpeptidergic-specific genes. Statistical analyses were done using two-way ANOVA with a Bonferroni post-test, N = 5 for M and P, N = 7 for the rest. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ns non-significant. Runx1f/f mice were used as control animals for analysis of Runx1 CKO mutants. Scale bar, 50 μm.