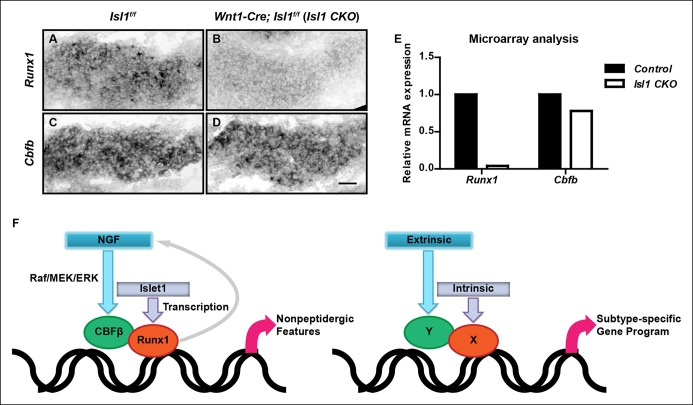
Figure 8. Islet1 is required for initiation of Runx1, but not Cbfb expression.
(A–D) In situ hybridization analysis of expression of Runx1 (A and B) and Cbfb (C and D) in control and Isl1 CKO DRGs at E12.5 shows that Islet1 deficiency abolishes expression of Runx1 but not Cbfb at an early age. Shown are representative images from two independent experiments. (E) Microarray analysis of E12.5 control and Isl1 CKO DRGs further confirms the differential dependence of expression of Runx1 and Cbfb on Islet1. Shown are average expression levels from two independent experiments that are normalized to the control levels for each gene. Expression levels have been normalized using globe scaling. Isl1f/f mice were used as control animals for analysis of Isl1 CKO mutants. (F) Schematics illustrating a molecular mechanism underlying specification of nonpeptidergic nociceptors and its general implication in the context of subtype specification. The extrinsic cue NGF and the intrinsic cue Islet1 coordinately regulate the Runx1/CBFβ complex, a nonpeptidergic nociceptor transcription factor complex, by preferentially targeting Cbfb and Runx1 for transcriptional regulation, respectively. Furthermore, the Runx1/CBFβ complex, through an unknown mechanism, enhances the level of NGF-TrkA signaling, resulting in a positive feedback loop between NGF-TrkA signaling and Runx1/CBFβ complex. This gene regulatory mechanism not only underscores the importance of interplay between extrinsic and intrinsic factors during multilineage differentiation, but also illustrates how such interplay can control cell-fate decisions through the convergence of extrinsic and intrinsic signals at the level of a heterodimeric, lineage-specific transcription factor complex. Scale bar, 50 μm.