Abstract
Background
The use of the immunosuppressive drug tacrolimus (TAC) is related to new onset diabetes after transplantation. Herein, we examined the effect of intraperitoneal administered TAC on intestinal glucose absorption in mice.
Methods
Animals received low, medium, or high dose TAC (0.5, 1, or 5 mg/kg/d, respectively), or 0.9% saline solution (control) for 14 days. Oral glucose tolerance test (OGTT), insulin concentration test, and serum TAC concentration measurements was performed after 14 days of TAC exposure. Plasma insulin was assessed and electrogenic glucose absorption were measured by the sodium-dependent increase of the short-circuit current. Expression levels of the glucose transporters sodium glucose co-transporter (SGLT) 1, glucose transporter (GLUT) 2, and GLUT5 were also determined.
Results
Oral glucose absorption assessed by OGTT was significantly enhanced in the low, medium, and high groups. Serum insulin was elevated in the medium and high group compared with the control. Moreover, glucose-induced Isc was significantly higher in TAC administrated groups, which indicates that SGLT1 activity increased. Transcription levels and protein abundance of SGLT1 in the experimental groups also increased compared with the control.
Conclusions
TAC induced insulin resistance and strengthened intestinal glucose absorption by increasing the activity and expression of the glucose transporter, SGLT1.
Introduction
New-onset diabetes after transplantation (NODAT) is a severe complication following organ transplantation and has been reported to occur in between 2% and 53% of transplanted patients [1]. Post-transplant hyperglycemia is related to risks of infection, cardiovascular disease [2], and graft dysfunction [3], and is associated with higher mortality [4]. Although there are a multitude of factors that contribute to the development of NODAT, the dominant factor is the use of immunosuppressive agents, such as corticosteroids, calcineurin-inhibitors (CNIs), and sirolimus, amongst others [5]. Tacrolimus (TAC) and cyclosporine are two widely used calcineurin-inhibitor drugs and both have diabetogenic effects [6]. The use of TAC is associated with an incidence of NODAT that is 2.5-fold greater than cyclosporine [1, 7, 8].
The mechanism underling the diabetogenic effect of TAC is currently being investigated [6, 9]. There are many studies focusing on changes in β-cell regeneration, insulin secretion, and insulin resistance after TAC administration [10–12]. Although glucose absorption in the intestines plays a crucial role in glucose homeostasis [13], it is unknown if intestinal glucose absorption is involved in the diabetogenic effect of TAC. According to a previous study, glucose malabsorption in the jejunum after TAC treatment was observed and the effect was dose-dependent [14]. Therefore, it is reasonable to hypothesize that intestinal glucose absorption participates in the diabetogenic TAC process. Thus, the aim of this study was to study the influence of different doses of TAC on glucose absorption in the intestine and to evaluate glucose homeostasis.
In the small intestine, glucose is mainly transported by sodium glucose co-transporter 1 (SGLT1), glucose transporter 2 (GLUT2), and GLUT5 [15]. SGLT1 transports glucose by coupling with Na+ and generates a short-circuit current (Isc) that can be measured by using an Ussing chamber [16]. Thus, to some extent, Isc represents the activity glucose transport by SGLT1. GLUT2 and GLUT5 are Na +-independent transporters that transport monosaccharides and fructose, respectively, from the intestinal lumen into enterocytes and are dependent on a concentration gradient [15]. Of these transporters, SGLT1 is the major route for the transport of sugars from the lumen into enterocytes. Therefore, we investigated changes in activity and expression of these glucose transporters, mainly focusing on SGLT1, to elucidate the pathophysiological mechanism TAC on intestinal glucose absorption.
Materials and Methods
Mice
Male, 8-week-old C57BL/6 mice (Shanghai Animal Center, Chinese Academy of Science, Shanghai, People’s Republic of China) weighing 22–25 g were used for the experiments. The mice were housed under a strict 12:12 hour light-dark cycles (lights on at 7 AM) at 20°C in standard pathogen-free conditions and allowed unrestricted access to standard rodent chow (Zhejiang Academy of Medical Sciences) and water. Every mouse was kept in a plastic box called an Individually Ventilated Cage (IVC) measuring 325 mm x 210 mm x 180 mm.
Animal in vivo procedures in this study were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at Zhejiang University, and the ethics committee of Zhejiang University also approved this study.
Groups and tissue harvest
A total of 24 C57BL/6 mice were used in the present study. By the completely random design, all mice were allocated into four groups according to TAC dose: 0.5 mg (low dose group), 1 mg (medium dose group), and 5 mg/kg (high dose group) for experimental groups, and 0.9% saline solution for the control group. Each group consisted of six mice.
TAC was supplied by Astellas Pharma, Inc. (Tokyo, Japan) and dissolved in 0.9% saline solution. Equivalent volumes of TAC and saline were administered intraperitoneally (i.p.) to the mice each morning for 14 days.
To minimize the influence of diurnal variety of glucose transporters [17], tissues from each group were harvested at 10 am. Mice were humanely euthanized via overdose with an intraperitoneal injection of sodium pentobarbitone anesthetic, according to standardized protocols supplied by the Zhejiang University’s Ethics Committee 14 days after continuous intraperitoneal injection. All procedures were performed at Zhejiang University’s Animal Experimentation Facility laboratories. After animals were anesthetized and sacrificed, blood samples were immediately collected through cardiac puncture and centrifuged at 3000 rpm for 15 minutes, and then the plasma was removed and frozen at −80°C until they were assayed.
Blood TAC levels were measured by immunoassay on an IMx analyzer (Abbott Diagnostics Laboratories, Abbott-Park, IL, USA) [18]. Since the study focused on the glucose absorption in the intestine, 4 cm jejunum segments at about 4 cm distal to the ligament of Treitz were collected. Next, 2 cm of this segment were used for Ussing chamber experiments. The remnant tissue was divided into two parts, one of which was snap frozen in liquid nitrogen and stored at –80°C, while the other was fixed in 10% formaldehyde for 24 hours, dehydrated, and embedded in paraffin.
Oral glucose tolerance test (OGTT) and insulin concentration tests
After fasting for 12 hours (9 pm to 9 am) with free access to water, mice were gavaged with D-glucose (3 g/kg body weight) in 0.9% saline solution. Blood samples were obtained from the tail vein before and after 20, 40, 60, and 80 minutes of glucose administration. Blood glucose was measured with a glucometer. Plasma insulin content was measured following glucose administration. At the above-mentioned time points, 100 μl of blood was collected [19] and plasma was isolated by low-speed centrifugation (2000 rpm, 5 minutes, 4°C). Plasma insulin was assessed by enzyme-linked immunosorbent assay (ELISA; Millipore, Watford, UK).
Histopathology
Intestinal morphology was assessed using our previously published method [16]. Briefly, 6-μm sections were made from tissues embedded in paraffin and stained with hematoxylin and eosin (H&E). The height and width of villus and crypt depth were measured in at least three animals and segments in each group. Villus surface was evaluated as π*(villus length * villus width) [20].
Electrophysiology
The jejunum segments were collected and incubated in ice-cold, gassed KRB solution containing 1 μM indomethacin for 10 minutes as previously described [21]. Briefly, the intestine was split longitudinally, and the seromusculature layers were removed and clamped into an Ussing chamber with an opening of 0.625 cm2. Under control conditions, the serosal and luminal perfusate contained (in mM): 105 NaCl, 2 KCl, 1 MgCl2, 1.25 CaCl2, 0.4 KH2PO4, 1.6 K2HPO4, 5 Na pyruvate, 25 NaHCO3, and 20 mannitol (pH 7.4, NaOH) [16]. The solutions were gassed with 95% O2 and 5% CO2 1 hour before the Ussing chamber experiment. The chamber temperature was maintained by a circulating water bath at 37°C. Next, the empty chamber was placed into the apparatus and the “potential difference” across the empty chamber set to “0 mV” before mounting the tissue into the Ussing chamber [22]. The basal level of the transepithelial potential difference (Vt) and the short-circuit current (Isc) was recorded continuously for 30 minutes, then 20 mM mannitol were replaced by 20 mM glucose in the luminal perfusate, and the recording continued for another 60 minutes. A further single jejunum segment from each group mice was harvested using the same preprocessing as above. The basal level of the transepithelial potential difference (Vt) and the short-circuit current (Isc) was recorded continuously for 30 minutes, then 20 mM mannitol were replaced by 20 mM glucose in the luminal perfusate, and the recording continued for another 30 minutes. Finally, 20 mM glucose were replaced by another 20 mM glucose solution simultaneously containing 100 μM/L LX-4211 (Lexicon pharmaceuticals) in the luminal perfusate, and the recording continued for another 60 minutes. The mean value was taken for final analysis. The results were expressed as the intensity of the Isc (μA/cm2) after glucose challenge over basal Isc.
Quantitative polymerase chain reaction
According to the methods of our previous study [16], total RNA was extracted from intestinal tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was reverse transcribed from total RNA (2 μg) using M-MLV Reverse Transcriptase (Promega, San Luis Obispo, CA, USA). Quantitative real-time PCR (qRT-PCR) was performed with an ABIPRISM 7500 Sequence Detection System (Applied Biosystems) using the SYBR Premix Dimer Eraser kit (Takara Biotechnology, Dalian, Liaoning, People’s Republic of China). With a total volume of 10 μl, amplification reactions, which included 1 μl cDNA template, 0.3 μl each of forward and reverse primers (10 μM), 0.2 μl of 50X ROX Reference Dye II (Takara), and 5 μl of 2X SYBR Premix Dimer Eraser. The primers were used as per our previous study [16]: sglt1 5ʹ-CAGTAACATTGGAAGTGGTCA-3ʹ (forward) and 5ʹ-GGGACAGAACGGAAAGGT-3ʹ (reverse), glut2 5ʹ-TTGCTGGACGAAGTGTATC-3ʹ (forward) and 5ʹ-GACTAATAAGAATGCCTGTGAC-3ʹ (reverse), glut5 5ʹ- CCACTGTCCATTGCTACC-3ʹ (forward) and 5ʹ-TTAGACATGCTCCTTTGATT-3ʹ (reverse), GAPDH 5ʹ-GGTGAAGGTCGGTGTGAACG-3ʹ (forward), and 5ʹ-CTCGCTCCTGGAAGATGGTG-3ʹ (reverse). Amplification of the transcripts involved an initial denaturation at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 55 C for 30 s, and 72°C for 34 s, and melting curve analysis was added after the final PCR cycle. The relative RNA expression was calculated using the delta-delta threshold cycle (ΔΔCT) method and normalized to GAPDH expression in the same cDNA sample.
Western blotting
Using our previously published method, proteins were extracted and concentrations determined [16]. Next, 20 μg of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking nonspecific binding sites for 2 hours in TBST (1X TBS, 0.1% Tween 20) containing 4% non-fat milk and 1% bovine serum albumin (BSA), the membranes were incubated overnight on ice with the primary antibodies against SGLT1 (EMD Millipore Corporation, Billerica, MA, USA; 1:3000), GLUT2 (EMD Millipore Corporation; 1:3000), GLUT5 (EMD Millipore Corporation; 1:3000) and β-actin (Sigma-Aldrich; 1:3000). After washing three times in TBST, the membranes were incubated for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies, and then visualized with enhanced chemiluminescence detection kit (Biological Industries, Beit Haemek, Israel) and intensities were quantified using Image-Pro plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
Data was analyzed using GraphPad-Prism 6 software (GraphPad-Prism Software Inc., San Diego, CA, USA) and provided as means ± standard error of the mean (SEM), where n represents the number of independent experiments. Differences between experimental groups and controls were assessed by Student t-test with or without Welch correction where applicable, and P < 0.05 was considered statistically significant.
Results
Body weight and food intake
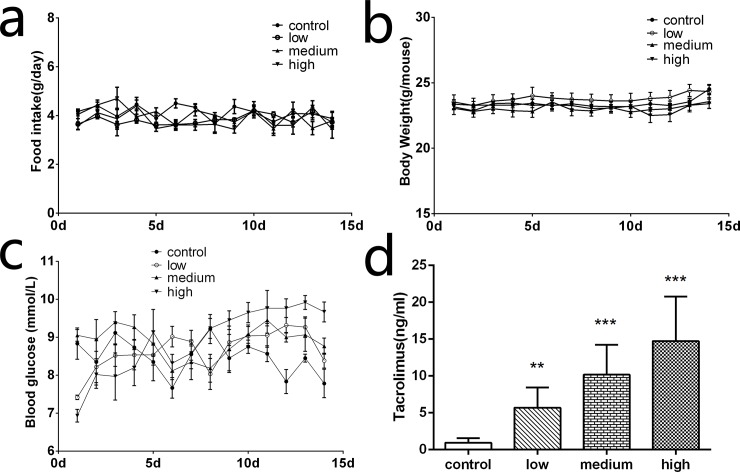
As the occurrence of clinical diabetes is often accompanied by changes in diet and weight, we monitored the food intake and body weight of each mice daily for 2 weeks. Surprisingly, food intake and body weight of mice during the observation remain stable and no significant difference was found between the four groups (Fig 1A and 1B).
Fig 1. Body weight, food intake, random blood glucose, and blood TAC concentration.
Body weight (a) and food intake (b) did not differ between groups in 14 days. (c) Random blood glucose was measured during the 14 experiment days. The use of TAC seemed to increase the blood glucose level and this effect was dose dependent. (d) Blood TAC concentration was dependent on TAC dose administrated in every group (n = 6, **P < 0.01, ***P < .001).
Random blood glucose
Random blood glucose of each mouse was monitored daily during the research period (Fig 1C). The control group maintained stable blood glucose levels. During 2 weeks of TAC administration, random blood glucose from the low and medium group rose slightly before the beginning of the experiment. In the high dose group, random blood glucose was obviously higher than basal levels, and also appeared higher level compared with the other three groups.
TAC blood concentration
After injecting TAC daily for 14 days, blood samples of each mouse were collected and blood TAC concentrations were measured. Blood concentration of TAC in low, medium, and high groups were compared with the control group, as shown in Fig 1D: low: 5.692 ± 1.219 ng/mL, P = 0.0052; medium: 10.18 ± 1.811 ng/mL, P = 0.001; high: 14.73 ± 2.705 ng/mL, P = 0.001; control: 0.9400 ± 0.2766 ng/mL. Among the groups, blood TAC concentration in the low and medium group were within the effective therapeutic concentration and equivalent to those seen in human transplant patients [23].
Oral glucose tolerance test (OGTT)
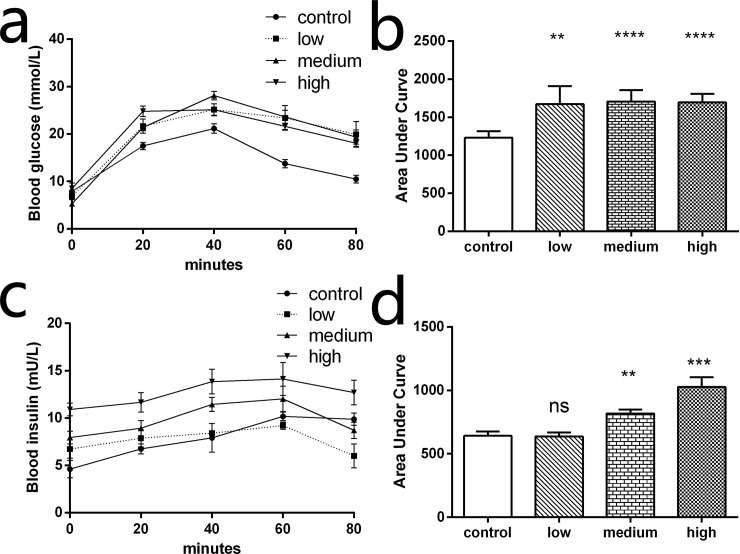
OGTT was performed to evaluate changes in glucose metabolism after TAC administration. As was shown in Fig 2A, the blood glucose levels of low, medium, and high groups were significantly higher than the control group at each time points during OGTT. Compared with the control group, the area under curve was significantly increased in low, medium, and high groups (P < 0.001; Fig 2B). This implies that TAC increased the absorption of glucose in intestines or reduced glucose metabolism.
Fig 2. Oral glucose tolerance test (OGTT) and insulin concentration test.
An OGTT (3 g/kg) was performed after drug administration for 14 days. (a) Blood glucose in TAC treated mice was significantly higher than those treated with saline during the test, indicating that glucose absorption increased after TAC administration. (b) The area under the curve for the low, medium and high group were also higher than control group. (c) Plasma insulin content was measured in mice following oral glucose administration. The medium and high dose groups displayed lowered glucose-stimulated insulin secretion. (d) The area under the curve for insulin releasing test show the medium and high dose groups were higher than the control group (n = 6, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns indicates no significant difference).
Using the insulin concentration test, we found increased levels of plasma insulin in the high and medium group compared with the control group (Fig 2C). Moreover, the area under curve was significantly higher in the medium (P = 0.0037) and high (P = 0.0008) groups compared to the control group (Fig 2D). No obvious difference was found between the low and control groups.
Electrogenic Glucose Transport
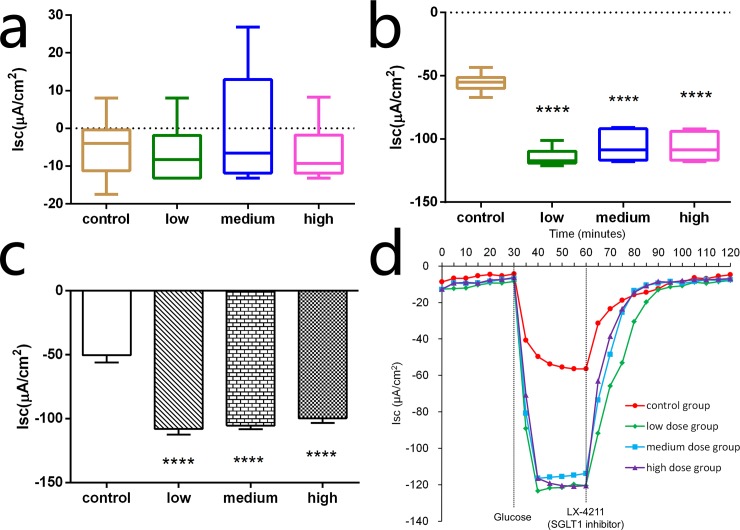
Electrophysiological analysis was used to evaluate changes in intestine glucose transportation after TAC administration. In the absence of glucose, basal Isc was not significantly different from the control group (–4.95 ± 3.40 μA/cm2), low group (–6.67 ± 3.30 μA/cm2), medium group (–0.43 ± 6.28 μA/cm2) or high group (–6.67 ± 3.20 μA/cm2) at any time point (Fig 3A); this revealed that the basal activity of SGLT1 among the four groups were equivalent. A rapid and marked lumen-negative increase of Isc was induced after partial iso-osmotic replacement of 20 mM mannitol by 20 mM glucose in the luminal side (Fig 3B). The decrease in Isc induced by glucose in low (–114.6 ± 2.93 μA/cm2, P < 0.0001), medium (–105.8 ± 4.89 μA/cm2, P < 0.0001), and high groups (–106.4 ± 4.54 μA/cm2, P < 0.0001) were significantly higher than the control group (–55.36 ± 3.12 μA/cm2). Delta Isc (ΔIsc) represented the decrease in Isc after glucose presentation and indicates the enhanced activity of SGLT1. As shown in Fig 3C, ΔIsc in low (–107.9 ± 4.56 μA/cm2, P < 0.0001), medium (–105.4 ± 2.97 μA/cm2, P < 0.0001) and high groups (–99.75 ± 3.52 μA/cm2, P < 0.0001) were significantly higher than in control groups (–50.41 ± 5.74 μA/cm2). Glucose transport was completely inhibited when 100 μM/L LX-4211 was added to the glucose solution (Fig 3D), suggesting dependence on SGLT1. These findings indicate that electrogenic glucose transport in the jejunum significantly increased after administration of TAC. In other words, TAC enhanced intestine glucose transport through SGLT1.
Fig 3. Electrogenic glucose transport.
(a) Basal Isc was not significantly different between groups. (b) The addition of 20 mM glucose on the luminal side of the Ussing chamber significantly decreased the negative Isc, which was significantly lower in the low, medium, and high dose groups than the control group. (c) The addition of 20 mM glucose induced a sharp decrease in Isc (ΔIsc). ΔIsc was significantly increased in the low, medium, and high dose groups compared with the control group (n = 6, ****P < 0.0001). (d) The assay repeated in the presence of 100 μM/L LX-4211, confirming glucose transport measured by the Ussing chamber represents SGLT1.
Histology
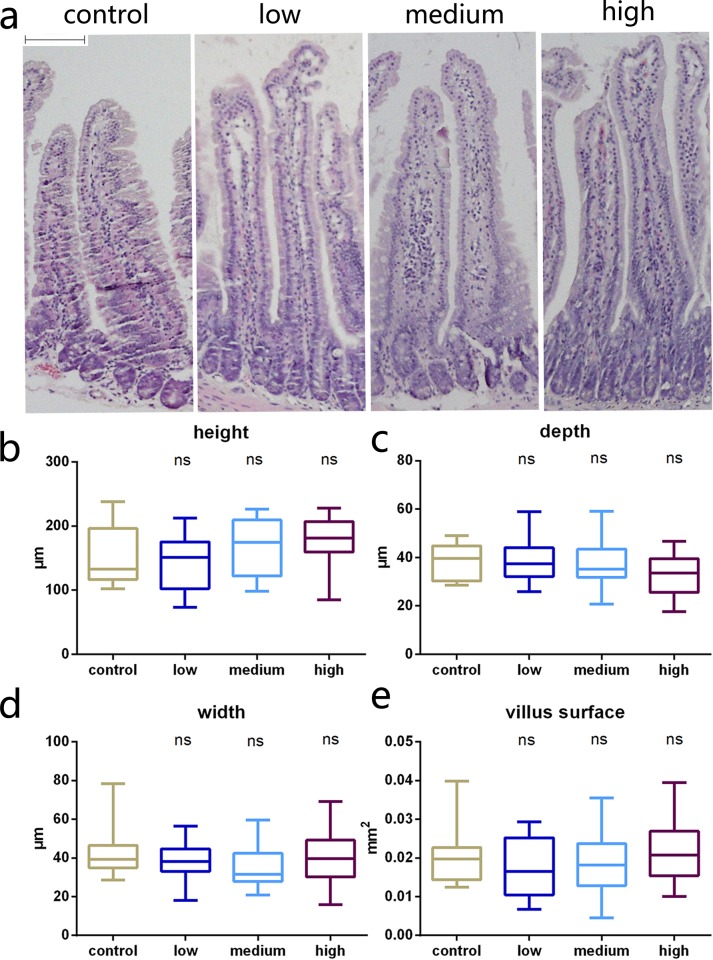
With respect to villi height and width, crypt depth, and the villus surface, no significant differences were observed between the four groups (Fig 4). This result suggests that TAC may have no effect on intestine morphology.
Fig 4. Intestinal histology.
(a) Micrographs of representative sections from the four groups of mice (control, low, medium, and high dose; hematoxylin and eosin, ×100). Histological comparisons of jejunal segments from the four groups show villus height (b), villus width (c), crypt depth (d), and villus surface (e) did not differ after receiving different TAC doses (n = 6, ns indicates no significant difference). Scale bar: 50 μm.
Transporter transcription and expression
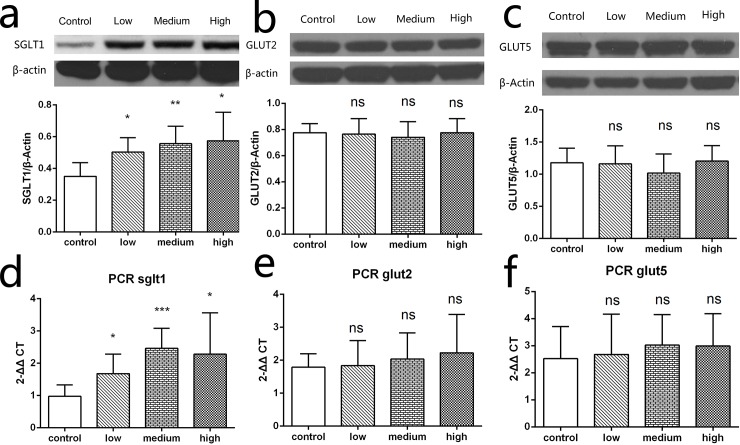
We further investigated gene transcription and protein expression levels of intestine glucose transporters, including SGLT1, GLUT2, and GLUT5. Consistent with the electrophysiology results, the relative transcription of sglt1 mRNA in low, medium, and high groups rose by 72% (P = 0.0339), 152% (P = 0.0005) and 134% (P = 0.037), respectively compared with the control group (Fig 5D). In accordance with mRNA results, the low, medium, and high groups showed significantly increased SGLT1 protein abundance compared with the control group (P = 0.013, P = 0.0047, and P = 0.0197, respectively; Fig 5A). However, both gene transcription levels and protein expression levels of GLUT2 (Fig 5B and 5E) and GLUT5 (Fig 5C and 5F) in the low, medium, or high groups were not significantly different from controls.
Fig 5. Transcription and expression of SGLT1, GLUT2, and GLUT5.
(d) Transcription of sglt1 was increased in low, medium, and high dose groups compared with the control group. Transcription of glut2 (e) and glut5 (f) did not changed after TAC administration. Protein abundance of SGLT1 was determined by Western blot normalized to β-actin. (a) SGLT1 expression in low, medium, and high dose groups were significantly higher than the control group. For GLUT2 (b) and GLUT5 (c) expression, no significant difference was observed between groups (n = 6, *P < 0.05, **P < 0.01, ***P < 0.001, ns indicates no significant difference).
Discussion
In the current study, we have found impaired glucose tolerance and increased plasma insulin levels in mice after TAC administration. Moreover, the Ussing chamber experiments showed that electrogenic glucose transport in the jejunum was significantly enhanced after TAC administration. We also revealed transcription and expression changes in SGLT1 after TAC administration, which represents a promising therapeutic target for new-onset diabetes after transplantation.
We also found that food intake and body weight were not significantly affected by TAC intake. The possible reason for this may be that 2 weeks of TAC administration was not long enough to generate typical chronic symptoms of diabetes, including increased food intake and weight loss. However, the OGTT showed that blood glucose levels were elevated after TAC administration in low, medium, and high dose groups. A reasonable explanation for this phenomenon is likely impairment of glucose tolerance and/or increasing intestinal glucose absorptive capacity.
The effect of TAC on plasma insulins level remains controversial. Previous studies have found that plasma insulin levels after TAC administration decreased due to the impairment of islet β-cell function mediated through depleting islet β-cell amount, or reducing insulin synthesis or release [11]. However, Weiguo et al. found markedly elevated insulin resistance levels in TAC-treated recipients following kidney transplantation, and illustrated a positive correlation between TAC concentration and insulin resistance [24]. A 2-year follow-up cohort study on kidney transplant patients found that blood insulin levels in NODAT were significantly elevated and insulin resistance was the primary cause of TAC-induced NODAT [25]. In our work, serum insulin and blood glucose levels were both elevated in the medium and high groups, which suggests that insulin sensitivity was impaired by medium and high, but not low TAC doses, showing a dose dependent TAC effect on insulin sensitivity and induce insulin resistance. This finding is in accordance with earlier research in rats [26]. Recently, Pereira et al. have found that GLUT4 has been removed from the cell surface via an increased rate of endocytosis with therapeutic concentrations of TAC and glucose uptake was inhibited independent of insulin signaling, which is a novel mechanism for how TAC contributes to the development of insulin resistance and diabetes [27]. According to our results and previous published studies, we think that hyperglycemia initially occurred due to an increase in intestinal glucose absorption when TAC was administrated, and sustained hyperglycemia led to increased insulin secretion. The mice gradually became resistant to the effects of insulin as the receptors that bind to the hormone become less sensitive to insulin concentrations resulting in hyperinsulinemia and disturbances in insulin release [28]. With a reduced response to insulin, the β-cells secrete increasing amounts of insulin in response to the continued high blood glucose levels resulting in hyperinsulinemia, which is defined as a condition in which there are excess levels of insulin circulating in the blood than expected relative to the level of glucose [29]. Toxic effects of hyperglycemia [30], toxicity of TAC [11], and excessive secretion by β-cells due to insulin resistance, all contributed to impairment of β-cell function, which eventually lead to hypoinsulinemia.
Interestingly, by comparing intestinal morphology, we found the villus, crypt, and the villus surface, which represent the absorptive area, were not significantly changed in any experiment group after TAC administration. Moreover, combined with the OGTT result, suggests that blood glucose elevation in the experiment groups were not due to the enlargement of intestinal absorption area. Reasonably, the discordance between the unchanged intestinal morphology and increased intestinal glucose absorption is potentially instructive.
We focused our research on SGLT1, rather than other glucose transporters. SGLT1 is the dominating glucose transporter in the intestine [31], thus we estimated glucose absorptive capacity by the Ussing chamber experiments. We found that electrogenic glucose transport in low, medium, and high groups was significantly enhanced after TAC administration. Furthermore, this increase was not significantly different between the three groups, which indicates that the enhancement of glucose transportation is not dependent on TAC dose. Meanwhile, in our studies, glucose transport measured by the Ussing chamber was almost completely inhibited by LX-4211, an inhibitor of SGLT1, which further supported the role of SGLT1 in glucose transport in the intestine. Much of the research about the effect of immunosuppressants on intestine absorption over the last two decades have examined the effect of TAC on intestine glucose absorption, which produced conflicting results. Some studies found that TAC had no direct and immediate influence on rat intestinal glucose absorption in therapeutic concentrations [32, 33]. However, another study revealed glucose malabsorption in the jejunum after 6 weeks of TAC treatment and the effect revealed dose dependency [14]. Interestingly, one study observed that glucose absorption increased in the jejunum and decreased in the ileum of rats chronically administrated with TAC [34].
Consistent with our electrophysiological results, the protein abundance of SGLT1 in the intestine was higher in experimental versus control groups. Although SGLT1 plays a pivotal role in intestinal glucose absorption [31], we also measured the transcription and expression of other glucose transporters in the gut, such as GLUT2 and GLUT5. The mRNA and protein level of these transporters did not change after TAC administration, irrespective of dose. Thus, we believe that the enhancement of intestine glucose transport resulted from increased expression of the glucose transporter SGLT1.
Throughout the entire experiment, our research focus was on glucose absorption in the intestines to research the mechanism of the diabetogenic effect in mice. First, we applied electrophysiological technology, the Ussing chamber, which is an apparatus for measuring epithelial membrane properties, and can quantify transportation and barrier functions of living tissue, such as measuring the short-circuit current as an indicator of net ion transport taking place across an epithelium. Compared with other methods, the Ussing chamber is more consistent with physiological condition in vivo. Moreover, we could conveniently perform inhibition tests, which makes our results more credible. Finally, we used molecular biology tests to verify the increased expression of SGLT1.
In conclusion, our study showed enhanced glucose absorption in the jejunum by upregulated expression of SGLT1 induced by TAC. Moreover, administration of TAC also resulted in insulin resistance. Taking together, as the main executor of intestinal glucose absorption, SGLT1 represents a potential therapeutic target for new-onset diabetes after transplantation.
Supporting Information
(XLSX)
(XLSX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Public Welfare Technology Application Research Plan of Zhejiang (no. 2015C33117), National S&T Major Project (no. 2012ZX10002017) and Foundation for Innovative Research Groups of the National Natural Science Foundation of China (No. 81121002).
References
- 1. Pham PT, Pham PC, Lipshutz GS, Wilkinson AH. New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am. 2007;36(4):873–90; vii. Epub 2007/11/07. doi: S0889-8529(07)00080-1 [pii]. 10.1016/j.ecl.2007.07.007 . [DOI] [PubMed] [Google Scholar]
- 2. Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69(3):588–95. Epub 2006/01/06. doi: 5000116 [pii]. 10.1038/sj.ki.5000116 . [DOI] [PubMed] [Google Scholar]
- 3. Valderhaug TG, Hjelmesaeth J, Jenssen T, Roislien J, Leivestad T, Hartmann A. Early posttransplantation hyperglycemia in kidney transplant recipients is associated with overall long-term graft losses. Transplantation. 2012;94(7):714–20. Epub 2012/09/12. 10.1097/TP.0b013e31825f4434 . [DOI] [PubMed] [Google Scholar]
- 4. Valderhaug TG, Hjelmesaeth J, Hartmann A, Roislien J, Bergrem HA, Leivestad T, et al. The association of early post-transplant glucose levels with long-term mortality. Diabetologia. 2011;54(6):1341–9. Epub 2011/03/17. 10.1007/s00125-011-2105-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kesiraju S, Paritala P, Rao Ch UM, Sahariah S. New onset of diabetes after transplantation—an overview of epidemiology, mechanism of development and diagnosis. Transpl Immunol. 2014;30(1):52–8. Epub 2013/11/05. 10.1016/j.trim.2013.10.006 S0966-3274(13)00086-5 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6. Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4(4):583–95. 10.1046/j.1600-6143.2003.00372.x . [DOI] [PubMed] [Google Scholar]
- 7. Lankarani KB, Eshraghian A, Nikeghbalian S, Janghorban P, Malek-Hosseini SA. New onset diabetes and impaired fasting glucose after liver transplant: risk analysis and the impact of tacrolimus dose. Exp Clin Transplant. 2014;12(1):46–51. Epub 2013/08/02. 10.6002/ect.2013.0047 . [DOI] [PubMed] [Google Scholar]
- 8. Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, et al. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(5):590–8. . [DOI] [PubMed] [Google Scholar]
- 9. Abe T, Onoe T, Tahara H, Tashiro H, Ishiyama K, Ide K, et al. Risk factors for development of new-onset diabetes mellitus and progressive impairment of glucose metabolism after living-donor liver transplantation. Transplantation proceedings. 2014;46(3):865–9. 10.1016/j.transproceed.2013.12.027 . [DOI] [PubMed] [Google Scholar]
- 10. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–61. Epub 2007/09/06. 10.1172/JCI32959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hjelmesaeth J, Asberg A, Muller F, Hartmann A, Jenssen T. New-onset posttransplantation diabetes mellitus: insulin resistance or insulinopenia? Impact of immunosuppressive drugs, cytomegalovirus and hepatitis C virus infection. Curr Diabetes Rev. 2005;1(1):1–10. Epub 2008/01/29. . [DOI] [PubMed] [Google Scholar]
- 12. Dong M, Parsaik AK, Eberhardt NL, Basu A, Cosio FG, Kudva YC. Cellular and physiological mechanisms of new-onset diabetes mellitus after solid organ transplantation. Diabet Med. 2012;29(7):e1–12. Epub 2012/03/01. 10.1111/j.1464-5491.2012.03617.x . [DOI] [PubMed] [Google Scholar]
- 13. Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2011;70(2):185–93. Epub 2011/04/01. 10.1017/S0029665111000103 . [DOI] [PubMed] [Google Scholar]
- 14. Malinowski M, Martus P, Lock JF, Neuhaus P, Stockmann M. Systemic influence of immunosuppressive drugs on small and large bowel transport and barrier function. Transpl Int. 2011;24(2):184–93. Epub 2011/01/07. 10.1111/j.1432-2277.2010.01167.x . [DOI] [PubMed] [Google Scholar]
- 15. Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN Journal of parenteral and enteral nutrition. 2004;28(5):364–71. . [DOI] [PubMed] [Google Scholar]
- 16. Yan S, Sun F, Li Z, Xiang J, Ding Y, Lu Z, et al. Reduction of intestinal electrogenic glucose absorption after duodenojejunal bypass in a mouse model. Obes Surg. 2013;23(9):1361–9. Epub 2013/04/16. 10.1007/s11695-013-0954-7 . [DOI] [PubMed] [Google Scholar]
- 17. Balakrishnan A, Stearns AT, Rounds J, Irani J, Giuffrida M, Rhoads DB, et al. Diurnal rhythmicity in glucose uptake is mediated by temporal periodicity in the expression of the sodium-glucose cotransporter (SGLT1). Surgery. 2008;143(6):813–8. Epub 2008/06/14. doi: S0039-6060(08)00187-6 [pii]. 10.1016/j.surg.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu S, Wu L, Jin J, Yan S, Jiang G, Xie H, et al. Influence of CYP3A5 gene polymorphisms of donor rather than recipient to tacrolimus individual dose requirement in liver transplantation. Transplantation. 2006;81(1):46–51. Epub 2006/01/20. doi: 00007890-200601150-00009 [pii]. . [DOI] [PubMed] [Google Scholar]
- 19. da Silva Xavier G, Mondragon A, Sun G, Chen L, McGinty JA, French PM, et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia. 2012;55(10):2667–76. Epub 2012/06/22. 10.1007/s00125-012-2600-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kisielinski K, Willis S, Prescher A, Klosterhalfen B, Schumpelick V. A simple new method to calculate small intestine absorptive surface in the rat. Clin Exp Med. 2002;2(3):131–5. Epub 2002/11/26. 10.1007/s102380200018 . [DOI] [PubMed] [Google Scholar]
- 21. Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1151–66. Epub 2009/04/04. 10.1152/ajpgi.90649.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rexhepaj R, Alesutan I, Gu S, Pelzl L, Eichenmuller M, Pathare G, et al. SGK1-dependent stimulation of intestinal SGLT1 activity by vitamin D. Pflugers Arch. 2011;462(3):489–94. Epub 2011/07/08. 10.1007/s00424-011-0987-5 . [DOI] [PubMed] [Google Scholar]
- 23. Storset E, Asberg A, Skauby M, Neely M, Bergan S, Bremer S, et al. Improved Tacrolimus Target Concentration Achievement Using Computerized Dosing in Renal Transplant Recipients-A Prospective, Randomized Study. Transplantation. 2015. 10.1097/TP.0000000000000708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sui W, Zou H, Zou G, Yan Q, Chen H, Che W, et al. Clinical study of the risk factors of insulin resistance and metabolic syndrome after kidney transplantation. Transpl Immunol. 2008;20(1–2):95–8. Epub 2008/08/16. 10.1016/j.trim.2008.07.003 S0966-3274(08)00053-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25. Chen QJ, Li J, Zuo SR, Zhang YP, Jia SJ, Yuan H, et al. Tacrolimus decreases insulin sensitivity without reducing fasting insulin concentration: a 2-year follow-up study in kidney transplant recipients. Renal failure. 2015:1–6. 10.3109/0886022X.2015.1007833 . [DOI] [PubMed] [Google Scholar]
- 26. Larsen JL, Bennett RG, Burkman T, Ramirez AL, Yamamoto S, Gulizia J, et al. Tacrolimus and sirolimus cause insulin resistance in normal sprague dawley rats. Transplantation. 2006;82(4):466–70. Epub 2006/08/24. 10.1097/01.tp.0000229384.22217.15 00007890-200608270-00005 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27. Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, et al. Cyclosporine A and tacrolimus reduce the amount of GLUT4 at the cell surface in human adipocytes: increased endocytosis as a potential mechanism for the diabetogenic effects of immunosuppressive agents. The Journal of clinical endocrinology and metabolism. 2014;99(10):E1885–94. 10.1210/jc.2014-1266 . [DOI] [PubMed] [Google Scholar]
- 28. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31 Suppl 2:S262–8. Epub 2008/02/15. 10.2337/dc08-s264 31/Supplement_2/S262 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29. Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M, et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest. 1985;75(3):809–17. Epub 1985/03/01. 10.1172/JCI111776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001;108(1):153–60. Epub 2001/07/04. 10.1172/JCI10294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorboulev V, Schurmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187–96. Epub 2011/11/30. 10.2337/db11-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Malinowski M, Martus P, Neuhaus P, Stockmann M. The influence of commonly used immunosuppressive drugs on the small bowel functions—a comparative experimental study. Ann Transplant. 2009;14(2):38–44. Epub 2009/06/03. doi: 883857 [pii]. . [PubMed] [Google Scholar]
- 33. Stockmann M, Engelmann BE, Langrehr JM, Neuhaus P. Influence of immunosuppressive drugs on intestinal epithelial transport function. Transplant Proc. 2002;34(5):1449–50. Epub 2002/08/15. doi: S004113450202924X [pii]. . [DOI] [PubMed] [Google Scholar]
- 34. Yanchar NL, Riegel TM, Martin G, Fedorak RN, Kneteman NM, Sigalet DL. Tacrolimus (FK506)—its effects on intestinal glucose transport. Transplantation. 1996;61(4):630–4. Epub 1996/02/27. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.