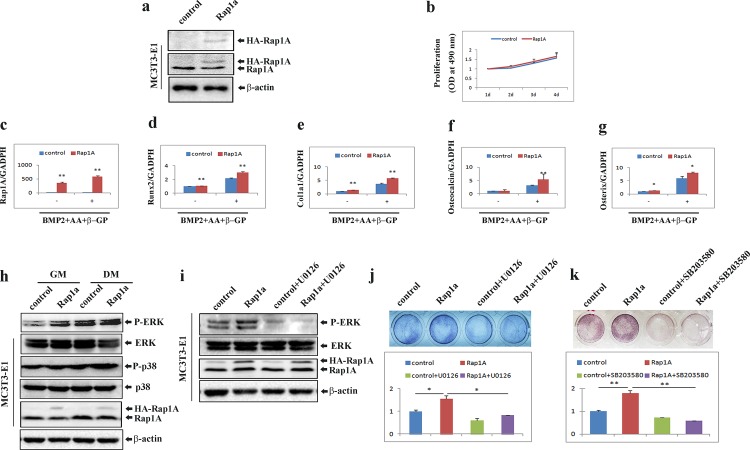
Fig 6. Forced expression of Rap1A accelerates osteoblast differentiation through activation of ERK1/2 and p38 MAPK.
(a) MC3T3-E1 cells stably expressing Rap1A were established as stated in the ‘‘Materials and Methods,” and Western blotting was used to test Rap1A expression with antibodies recognizing HA and Rap1A. (b) Proliferation of MC3T3-E1 cells with or without Rap1A overexpression tested by MTT assays. (c-g) Over-expression of Rap1A in MC3T3-E1 cells increased osteoblast-specific gene expression. (h) Western blot analyses with antibodies recognizing phospho-ERK1/2, total ERK1/2, phospho-p38, total p38 and Rap1A were performed in cells treated with either growth medium (GM) or osteoblast differentiation medium (DM) containing rhBMP-2 (100 ng/ml), ascorbic acid (50 μg/ml), and β-glycerophosphate (10 mM) for 3 days. β-actin was used as a loading control. (i) phospho-ERK1/2, total ERK1/2 and Rap1A were detected by Western blot in MC3T3-E1 cells with or without Rap1A overexpression after treatment with osteoblast differentiation medium (DM) for 3 days in the presence or absence of 10 μM U0126. β-actin was used as a loading control. (j and k) Inhibition of ALP activity by U0126 and SB203580 in cells with or without Rap1A overexpression after differentiation induction by osteogenic medium containing rhBMP-2 (100 ng/ml), ascorbic acid (50 μg/ml), and β-glycerophosphate (10 mM) for 3 days in the presence or absence of 10 μM U0126 (j) or 10 μM SB203580 (k). Cells were fixed and stained for ALP. ALP activities were measured by densitometry at 520 nm (below). Data represent means ± SD of triplicate samples. *, P < 0.05; **, P < 0.01.