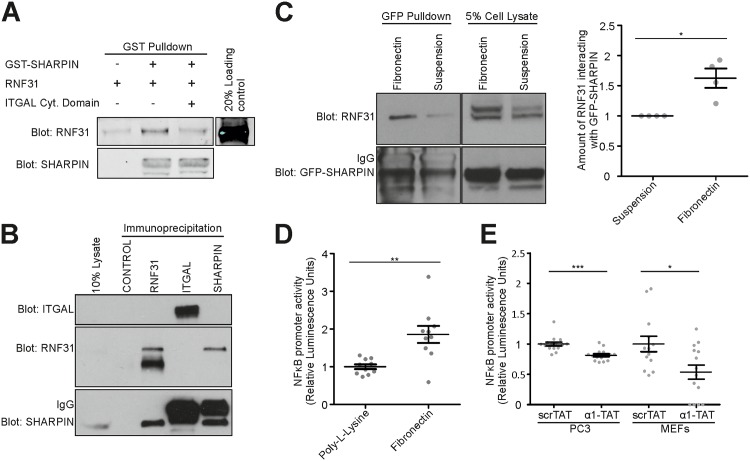
Fig 6. Integrin and RNF31 binding to SHARPIN are mutually exclusive.
(A) Pull-down assay showing that the presence of a peptide corresponding to the cytoplasmic domain of ITGAL prevents interaction between GST-SHARPIN and RNF31. (B) Co-immunoprecipitation of endogenous SHARPIN, RNF31 and ITGAL from Jurkat cells (a GFP antibody was used as negative control). (C) Pull-down of GFP-SHARPIN from HEK293 cells demonstrated that cells in suspension show decreased RNF31-SHARPIN interaction compared to adherent cells (n = 4). RNF31 binding was normalized to total RNF31 levels and the amount of pulled-down GFP-SHARPIN. (D) TNF-induced NF-κB promoter activity of PC3 cells, adherent to either 5 μg/ml fibronectin or poly-L-lysin (a substratum for integrin-independent cell adhesion), was measured using a luciferase reporter assay (n = 2 with 5 replicates each). (E) TNF-induced NF-κB promoter activity of PC3 cells or WT MEFs, incubated with a membrane-permeable ITGA1-tail peptide (α1-TAT) or a scrambled peptide (ScrTAT), was measured using a luciferase reporter assay (n = 2 with 6–9 replicates, and n = 3 with 3–5 replicates, respectively). All numerical data are mean ± s.e.m. ***: p<0.001, **: p<0.01, *: p<0.05.