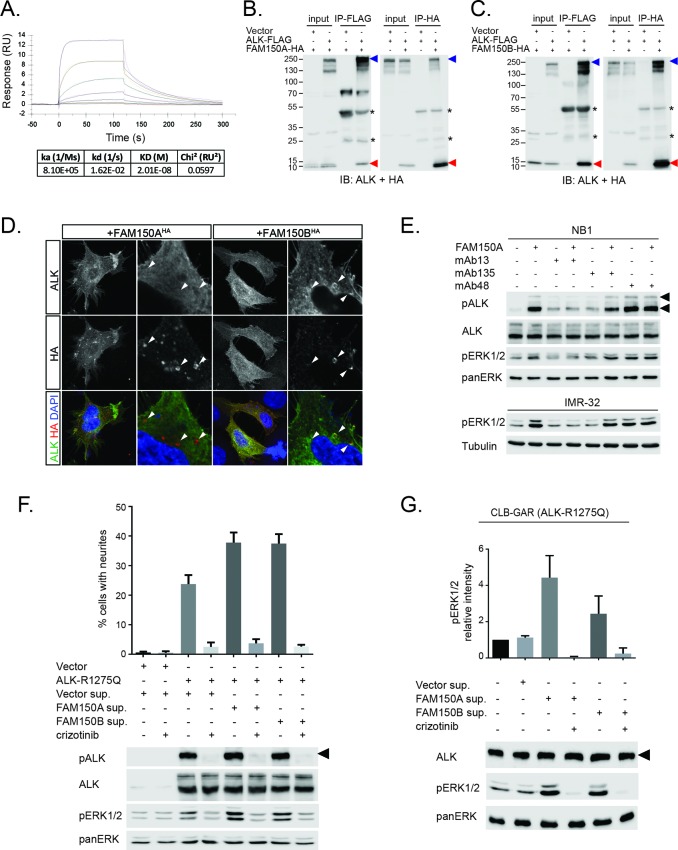
Figure 4. FAM150A and FAM150B bind to ALK and further activate signaling mediated by the R1275Q ALK neuroblastoma mutation.
(A) Binding kinetics of purified FAM150A to extracellular domain of anaplastic lymphoma kinase (ALK-ECD-Fc) in a Biacore surface plasmon resonance (SPR) analysis. (B) FAM150A immunoprecipitates with human ALK. Immunoprecipitation with either anti-FLAG(DYKDDDDK)(ALK) or anti-HA (FAM150A) was performed and the resulting immunoprecipitates immunoblotted for the presence of ALK (blue arrowheads) and FAM150A (red arrowheads), *indicates immunoglobulin light and heavy chains. (C) FAM150B immunoprecipitates with human ALK. Immunoprecipitation with either anti-FLAG (ALK) or anti-HA (FAM150B) was performed and the resulting immunoprecipitates immunoblotted for the presence of ALK (blue arrowheads) and FAM150B (red arrowheads),*indicates immunoglobulin light and heavy chains. (D) Human Embryonic Kidney (HEK) 293 cells expressing ALK were incubated with either control or HA-tagged FAM150A or HA-tagged FAM150B conditioned medium prior to analysis by immunohistochemistry. Both HA-tagged FAM150A and HA-tagged FAM150B bind to ALK-expressing cells. Higher magnification panels indicate intracellular vesicles positive for both ALK and HA-tagged FAM150A/B. (E) NB1 and IMR32 neuroblastoma cells were treated with 2 µg/ml monoclonal antibodies (mAB13, mAb48 or mAb135) prior to stimulation with FAM150A. Detection of ALK activation was visualized with pALK-Y1604 (arrowheads) and pERK1/2 in whole cell lysates. Pan-ERK or tubulin were employed for equal loading. (F) Whole cell lysates from PC12 cells expressing either vector control or ALK-R1275Q were stimulated with medium from HEK293 cells transfected with vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK activation was visualized with pALK-Y1604 (arrowheads) and pERK1/2 in whole cell lysates. Pan-ERK was employed for equal loading. Neurite outgrowth was performed in triplicate and each sample within an experiment was performed in duplicate (error bars indicate SD). (G) CLB-GAR cells harboring the ALK-R1275Q mutant were stimulated for 30 min with medium from HEK293 cells transfected with either vector control, FAM150A or FAM150B prior to analysis by immunoblot. Analysis was carried out in the presence or absence of 250 nM crizotinib. Detection of ALK signaling activation was visualized with pERK1/2. Pan-ERK was employed for equal loading. pERK1/2 intensity was analyzed from three independent experiments (error bars indicate SD).
Figure 4—figure supplement 1. FAM150A binds to the extracellular domain of human ALK by ELISA.
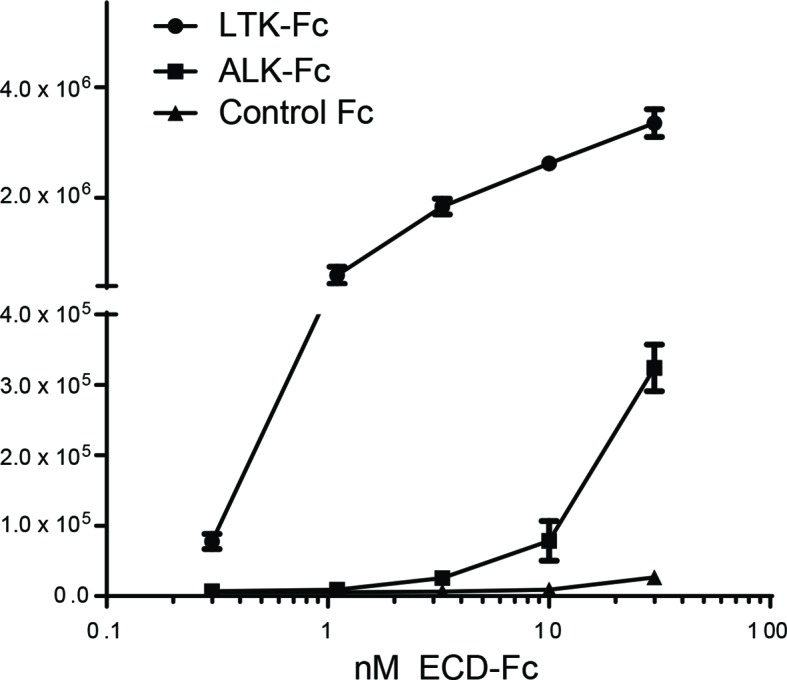
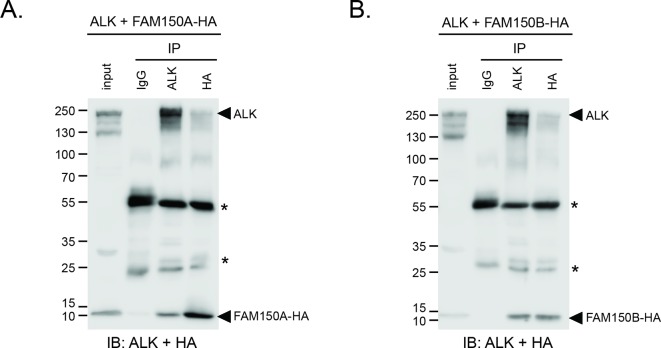
Figure 4—figure supplement 2. ALK interacts with both FAM150A and FAM150B.
Figure 4—figure supplement 3. ALK and LTK interact in the presence of FAM150A. FAM150A and anaplastic lymphoma kinase (ALK) coimmunoprecipitate with leukocyte tyrosine kinase (LTK).
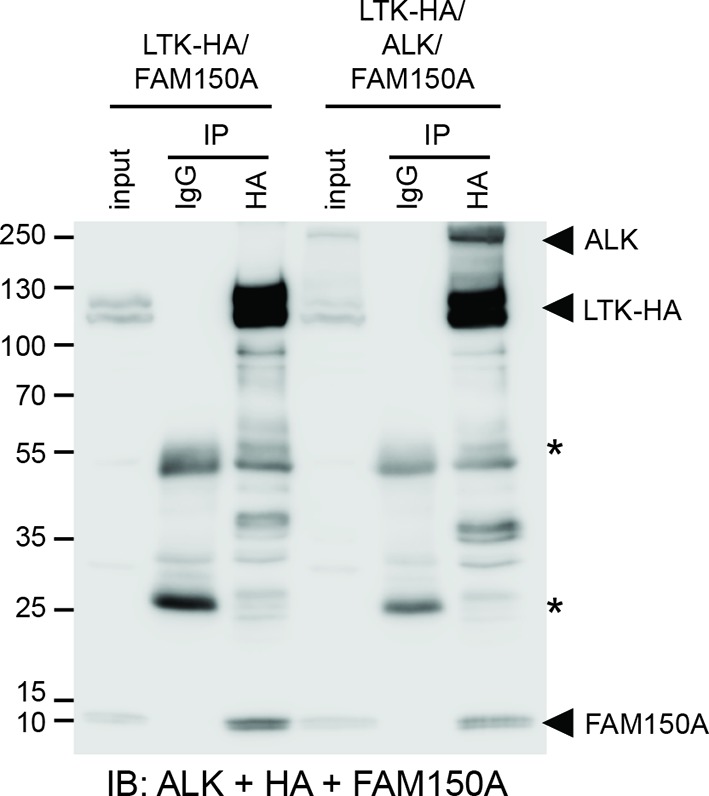
Figure 4—figure supplement 4. Effect of deletion of the glycine rich domain of ALK on FAM150A and FAM150B binding.
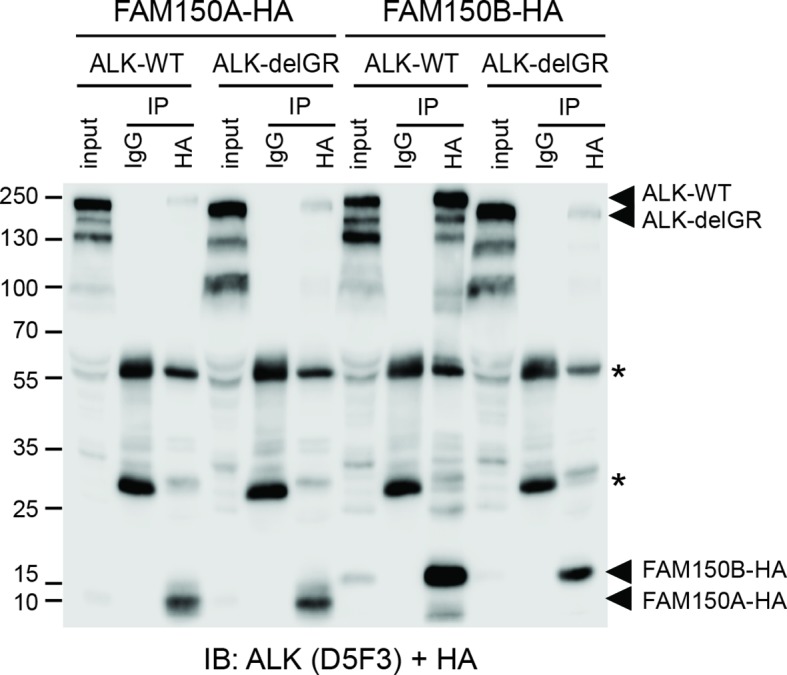
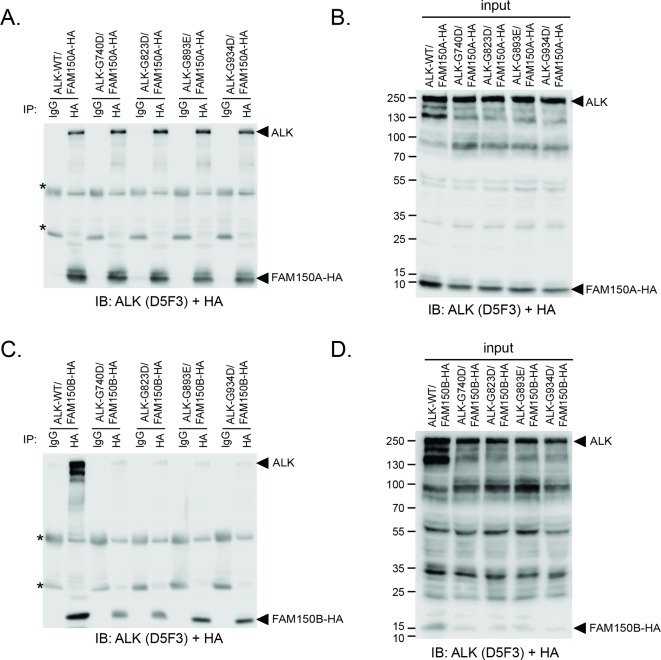
Figure 4—figure supplement 5. Effect of glycine mutations in the glycine-rich domain of ALK on FAM150A and FAM150B binding.
Figure 4—figure supplement 6. Identification of monoclonal antibodies recognising the glycine-rich region of the ALK ECD.
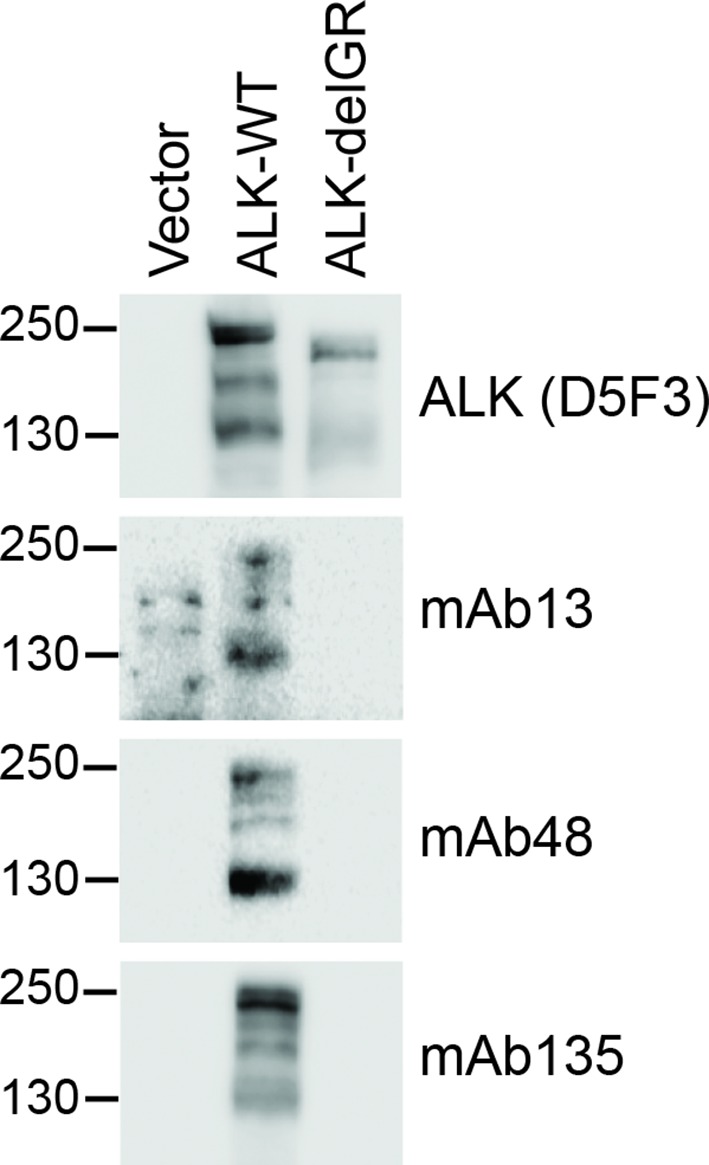
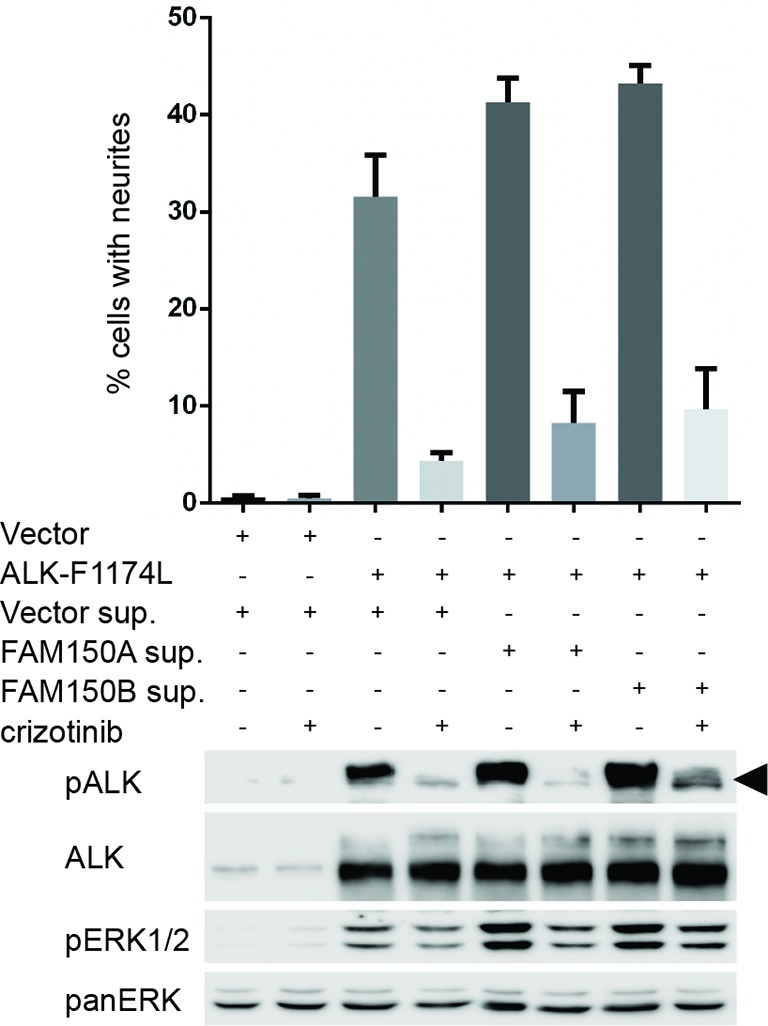
Figure 4—figure supplement 7. FAM150A and FAM150B bind to ALK and further activate signaling mediated by the ALK-F1174L neuroblastoma mutant.