Abstract
Objective
Physical pain and negative affect have been described as risk factors for alcohol use following alcohol treatment. The current study was a secondary analysis of two clinical trials for alcohol use disorder (AUD) to examine the associations between pain, negative affect and AUD treatment outcomes.
Method
Participants included 1383 individuals from the COMBINE Study (COMBINE Study Group, 2003; 31% female, 23% ethnic minorities, average age=44.4 (SD=10.2)), a multisite combination pharmacotherapy and behavioral intervention study for AUD in the United States, and 742 individuals from the United Kingdom Alcohol Treatment Trial (UKATT Research Team, 2001; 25.9% female, 4.4% ethnic minorities, average age=41.6 (SD=10.1)) a multisite behavioral intervention study for AUD in the United Kingdom. The Form-90 was used to collect alcohol use data, the Short Form Health Survey and Quality of Life measures were used to assess pain, and negative affect was assessed using the Brief Symptom Inventory (COMBINE) and the General Health Questionnaire (UKATT).
Results
Pain scores were significantly associated with drinking outcomes in both datasets. Greater pain scores were associated with greater negative affect and increases in pain were associated with increases in negative affect. Negative affect significantly mediated the association between pain and drinking outcomes and this effect was moderated by social behavior network therapy (SBNT) in the UKATT study, with SBNT attenuating the association between pain and drinking.
Conclusion
Findings suggest pain and negative affect are associated among individuals in AUD treatment and that negative affect mediated pain may be a risk factor for alcohol relapse.
Keywords: Alcohol use disorder (AUD) treatment, pain interference, pain intensity, negative affect, alcohol relapse
Introduction
Alcohol relapse, defined as the process of returning to heavy drinking (often defined as 4+/5+ drinks per occasion for women/men) after a period of abstinence or reduced alcohol use, is common in those with a history of alcohol use disorder (AUD), a diagnosis often associated with periods of heavy drinking and consequent negative impacts of drinking on functioning (Leshner, 1997; Sutton, 1979). The accurate identification of factors that increase the risk of relapse is of crucial importance and there have been widespread efforts to identify the relevant risk factors in order to improve relapse prevention interventions (Connors, Maisto, & Donovan, 1996; Moos & Moos, 2006; Witkiewitz & Marlatt, 2004).
Nearly all models of the relapse process have proposed an interaction between biological, psychological, environmental, and social factors, with an emphasis on more stable risk factors (i.e., distal or tonic risk; Shiffman, 1989; Witkiewitz & Marlatt, 2004) creating heightened vulnerability for relapse in the presence of more immediate risk factors (i.e., proximal or phasic risk; Shiffman, 1989; Witkiewitz & Marlatt, 2004). For example, a recent analysis by Chow and colleagues (2013) found that individuals who were high in distal risk were significantly more likely to experience a lapse if proximal risk (characterized by negative emotions, perceived stress, and craving) was heightened within the first two weeks of treatment. Similar studies of proximal risks such as dependence severity, emotional distress, and social support and recovery resources provide further support for proximal risks’ potential role in increasing or decreasing the probability of relapse to problematic alcohol use (e.g., Garland, Franken, & Howard, 2012; Moos & Moos, 2006; Witkiewitz & Villarroel, 2009; Witkiewitz, 2011). Despite the identification of multiple risk factors, very few studies—and few theoretical models—have acknowledged the common experience of physical pain and pain interference as potential predictors of alcohol treatment outcomes (Booker, Haig, Geisser, & Yamakawa, 2003; Witkiewitz, Vowles, McCallion, Frohe, Kirouac, & Maisto, in press). This gap in the literature is surprising given evidence supporting the relationship between pain and negative affect (e.g., Davis, Zautra, & Smith, 2004), as well as the association between negative affect and alcohol use (Witkiewitz & Villarroel, 2009). Furthermore, alcohol has historically been used for its analgesic affect (Trafton, Oliva, Horst, Minkel, & Humphreys, 2004; Woodrow & Eltherington, 1988) and individuals with AUD have identified pain as both a common experience and as a primary reason for alcohol use (Caldeiro et al., 2008). Given these findings, it is plausible that an important association may be present between the experience of pain and AUD treatment outcomes (Witkiewitz et al., in press). The relation between pain and AUD treatment outcomes may also be explained by experiences of negative affect.
Exploring this possibility was the primary objective of the present analyses. Specifically, the association between pain, negative affect, and alcohol treatment outcomes was examined via secondary analyses of data from two randomized clinical trials for alcohol dependence: the COMBINE Study (COMBINE Study Group, 2003), a large multisite randomized clinical trial for alcohol dependence conducted in the US, and the United Kingdom Alcohol Treatment Trial (UKATT Research Team, 2001), a large randomized clinical trial for alcohol problems conducted in the United Kingdom (UK). It was hypothesized that pain would be associated with alcohol treatment outcomes (Hypothesis 1) and negative affect (Hypothesis 2) and that negative affect would mediate the association between pain and drinking outcomes (Hypothesis 3). Additional exploratory analyses were conducted to determine whether the treatments received in the COMBINE or UKATT studies had any influence on pain, as well as whether treatment moderated any of the associations between pain, negative affect, and alcohol treatment outcomes.
Methods
COMBINE Participants and Procedures
The COMBINE study (“Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence;” COMBINE Study Group, 2003) randomized 1383 subjects across 11 research sites into 9 treatment groups, consisting of a combination of medical management (MM) or combined behavioral intervention (CBI) and medications (acamprosate, naltrexone, or placebo versions of each drug). Subjects received treatment for a total of four months; participants were offered 9 MM visits and a maximum of 20 CBI sessions. Participants completed assessments at 2.5 months, 9 months, and 12 months following treatment.
The sample was recruited from treatment referrals at the study sites and throughout the community, as described previously (Anton et al., 2006). Individuals were excluded from participation if they were dependent on another drug besides alcohol, nicotine, or cannabis, had recently used opioids, had a serious mental illness, or any other medical condition that could disrupt study participation, had taken one of the study medications 30 days prior to baseline, or took medication that could raise the potential risks of the study. Inclusion criteria required participants have a minimum of 14 drinks (females) or 21 drinks (males) on average per week over a successive 30-days in the 90-day period prior to beginning abstinence, having two or more days of heavy drinking (defined as 4+ drinks for females and 5+ drinks for males) in the 90-day period with the last drink being within 21 days of enrollment.
Of the 1,383 participants in COMBINE, 31% were female and 69% were male, 23% of the study patients were ethnic minorities (76.3% Non-Hispanic White, 11.6% Hispanic American, 7.8% African American, and 4.1% “other”). The participants’ mean age was 44.4 years (SD = 10.2), 71% had at least 12 years of education, and 42% were married.
UKATT Participants and Procedures
For UKATT (UKATT Research Team, 2001), 742 subjects across seven treatment sites in Birmingham, Cardiff, and Leeds were randomized into two treatment groups: either motivation enhancement therapy (MET) or social behavior and network therapy (SBNT; UKATT Study Research Team, 2005). Subjects received treatment for a total of two to three months; participants receiving SBNT received up to 8 sessions and those who received MET received up to 3 sessions. Participants completed assessments at 3- and 12-months after entry into the trial.
Participants were recruited into the study if they were seeking alcohol treatment from one of the treatment sites (UKATT Research Team, 2001, 2005). Individuals were excluded from participation if abuse or dependence of other drugs besides alcohol was a more serious problem, had uncontrolled psychotic symptoms or severe cognitive impairment, could not name a contact person, were planning to leave the area, were under 16 years old, or were illiterate.
Of the 742 participants in UKATT, 25.9% were female and 74.1% were male, 95.6% of the participants were White and 4.4% of the study patients were ethnic minorities (1.2% Black, 2.0% Indian or other Asian, 0.1 Pakistani, and 0.9% “other”). The mean age was 41.6 years (SD = 10.1), 10% had a secondary degree or equivalent training, and 44% were married.
Measures
Alcohol use
In both COMBINE and UKATT, alcohol consumption was assessed using a calendar method via the Form-90 Interview (Miller, 1996). In COMBINE daily alcohol consumption, measured in US standard drink units (unit of alcohol = 11.358 grams), was assessed for 485 days (120 days during treatment and 365 days following treatment). In UKATT daily alcohol consumption, measured in UK standard drink units (unit of alcohol = 8 grams of ethanol), was assessed for the prior 90 days at baseline, 3-month follow-up (corresponding to the post-treatment assessment), and the 12-month follow-up. Four outcome variables were derived from the Form-90 data in each of the studies: percent drinking days (PDD; a measure of drinking frequency), drinks per drinking day (DDD; a measure of drinking intensity), percent heavy drinking days (PHDD; defined as percentage of days with 4+/5+ drinks for women/men), and the maximum number of drinks on the peak drinking occasion (MXD, a measure of peak drinking).
Pain
In COMBINE, pain was assessed via two items. One item was from the 26 item World Health Quality of Life (WHO-QOL) assessment (World Health Organization, 1998): “To what extent do you feel that physical pain prevents you from doing what you need to do?” with response options ranging from 1 “not at all” to 5 “an extreme amount.” The second item was from the 12 item Short Form Health Survey (SF-12; Ware, Kosinski, & Keller, 1996): “During the past four weeks, how much did pain interfere with your normal work including both work outside the home and housework?” with response options ranging from 1 “not at all” to 5 “extremely.” The WHO-QOL measure was administered at baseline, 6.5 months post baseline, and 12 months post baseline. The SF-12 was administered at baseline, four months post baseline, and 12 months post baseline. For both items we found that the greatest pain interference categories of “an extreme amount” (WHO-QOL) and “extremely” (SF-12) had very low response rates (less than 1% indicated this level of pain interference). To address this, we recoded the response options for the WHO-QOL to be from 1 “not at all” to 4 “very much or an extreme amount” and the response options for the SF-12 to be from 1 “not at all” to 4 “quite a bit or extremely.” Internal consistency reliability of the two items exceeded α = 0.81 at all assessment time points.
In UKATT, pain was assessed via three items including one item from the European Quality of Life Group EQ-5D (European Quality of Life Group, 1990), a general measure of health status on five dimensions, and two items from the 36 item Short Form Health Survey (SF-36, Ruta, Garratt, Abdalla, Buckingham, & Russell, 1993). One item, the same item that was included in the SF-12 from COMBINE, measured pain interference: “During the past four weeks, how much did pain interfere with your normal work including both work outside the home and housework?” with response options ranging from “not at all” to “extremely.” Two items assessed pain intensity, one from the EQ-5D: “Please indicate which statement best describes your health today” with response options of “I have no pain or discomfort,” “I have moderate pain or discomfort,” or “I have extreme pain or discomfort” and one from the SF-36: “How much bodily pain have you had during the past 4 weeks” with response options ranging from “none” to “very severe.” The EQ-5D and SF-36 were administered at baseline, 3-months, and 12-month follow-ups. Internal consistency reliability of the three items was α = 0.77 at baseline and exceeded α = 0.81 at 3- and 12-month time points.
Negative Affect
In COMBINE, negative affect was measured by the Depression and Anxiety domains of the 53-item Brief Symptom Inventory (BSI; Derogatis, 1983), which assesses self-reported psychiatric symptomatology across nine domains via ratings on a 5-point scale (0 = “not at all”, 4 = “extremely”). The BSI was administered at baseline as well as post-baseline months four (end of treatment), 6.5 (2.5-months post-treatment, 12 (9-months post-treatment) and 16 (12-months post-treatment). Scores on the Depression and Anxiety domains were used as indicators of a negative affect latent variable at each time point. Internal consistency reliability of the BSI exceeded α = 0.97 at all assessment time points.
In UKATT, negative affect was measured by the Depression and Anxiety/Insomnia domains of the 28 item General Health Questionnaire (GHQ-28; Goldberg, 1972), which is a measure of psychiatric disturbance in four domains: Somatic Symptoms, Anxiety/Insomnia, Social Dysfunction, and Depression. The GHQ was administered at baseline, 3-months, and 12-month follow-ups. Scores on the Anxiety/Insomnia and Depression domains were used as indicators of a negative affect latent variable assessed at baseline, 3-months (post-treatment), and 12-month follow-up. Internal consistency reliability of the GHQ exceeded α = 0.96 at all assessments time points.
Covariates
Acknowledging that other factors are often associated with pain, negative affect, and alcohol treatment outcomes; we included a number of covariates in the final mediation models. Covariates were not included in the models of associations between pain and drinking outcomes or in the models of associations between pain and negative affect because we were primarily interested in the bivariate associations among these constructs, without the additional influence of covariates. Covariates in both the COMBINE and UKATT studies included: demographic variables (gender, marital status, employment, income, and minority status), baseline dependence severity [assessed via the Alcohol Dependence Scale (ADS; Skinner & Horn, 1984) in COMBINE and the Leeds Dependence Questionnaire (LDQ; (Raistrick et al., 1994) in UKATT], number of alcohol dependence symptoms based on the Diagnostic and Statistical Manual for Mental Disorders – Fourth Edition Text Revision (DSM-IV-TR; American Psychiatric Association, 1995), readiness to change [assessed using the University of Rhode Island Change Assessment scale (URICA; DiClemente & Hughes, 1990) in COMBINE and the Readiness to Change Questionnaire – Treatment Version (RTCQ; Heather & Honekopp, 2008) in UKATT], and self-efficacy was assessed using the Alcohol Abstinence Self-Efficacy Scale (AASE; DiClemente, Carbonari, Montgomery, & Hughes, 1994). In UKATT, which did not exclude opiate users (n=67, 9.0% of the UKATT sample), we included opiate use as a covariate.
Statistical Analyses
Analyses were conducted to test the three hypotheses listed above regarding the association between pain, negative affect and alcohol treatment outcomes. Models were estimated in the COMBINE and UKATT datasets using Mplus version 7.2 (Muthén & Muthén, 2012) with maximum likelihood estimation, which is a preferred method for estimation when some data are missing (Schafer & Graham, 2002). These models were considered to provide a reasonable fit to the data with a Root Mean Square Error of Approximation (RMSEA; Browne & Cudeck, 1993) < 0.08 and the Comparative Fit Index (CFI; Bentler, 1990) > 0.90. There are varying opinions on the best cut-offs for the RMSEA and CFI for evaluating model fit, with some arguing for RMSEA < 0.06 and CFI > 0.95 as indicative of good fit (Hu & Bentler, 1999). We believed it was important not to focus exclusively on fit indices (although model fit is important) and to consider the replication of models across two samples as the ultimate goal.
Measurement Models of Pain and Negative Affect
First, we created longitudinal measurement models of the pain and negative affect constructs within each dataset. The underlying measurement model for each construct was selected based on the distributions of the data available, with graded response models used for ordered categorical items and confirmatory factor analysis used for continuous scale scores.
For the pain items, which were ordered categorical, we used longitudinal graded response models (Samejima, 1969; 1997) to create individual graded response scores at each time point using the expected a posteriori approach. Separate graded response models were estimated in the COMBINE and UKATT studies in order to generate graded response scores (similar to factor scores) for the pain latent factors at each time point (COMBINE: baseline, 4-, 6.5-, and 12-months post baseline; UKATT: baseline, 3-, and 12-months post baseline).
For negative affect variables, which were continuous scale scores, we used longitudinal confirmatory factor analyses (Little, Preacher, Selig, & Card, 2007; Little, 2013) to create study-independent latent factor scores at each time point. Longitudinal confirmatory factor analysis is a repeated measures extension of the confirmatory factor analysis model, whereby factor loadings and item intercepts are constrained to equality across time. First, we estimated latent factor models of negative affect at each time point (COMBINE: baseline, and 4-, 6.5-, 12-, and 16-months post baseline; UKATT: baseline and 3-, and 12-months post baseline) and then combined them into a longitudinal model with constraints placed on the item loadings, intercepts, and variance-covariance matrix to test for measurement invariance of the latent factor across time (Byrne, Shavelson, & Muthén, 1989). Models were estimated separately in the COMBINE and UKATT studies in order to generate factor scores for the negative affect latent factors at each time point.
For each dataset we used the pain graded response scores and negative affect latent factor scores as indicators in latent growth curve models of changes in pain and negative affect, respectively, over time. Parameters derived from a latent growth model provide information about a construct’s average level (mean intercept) and average change over time (mean slope), as well as the individual variance around the intercept and slope. A significant intercept would indicate an average level that is significantly different from zero, whereas a significant slope would indicate an increase or decrease in either pain or negative affect score that was significantly different from no change. In both datasets we set the intercept for the growth models at the end of treatment (COMBINE: 4-month assessment; UKATT: 3-month assessment) and we examined the fit of models using varying parameterizations of the growth process (linear, quadratic, nonlinear). Linear growth represents the average rate of change in pain and negative affect scores over time, quadratic growth represents acceleration or deceleration in the rate of change (e.g., the change is more rapid or slowed, introducing a parabolic change pattern), and nonlinear growth models could be used to identify other functional forms of growth (e.g., exponential growth).
Hypothesis 1: Association between Pain and Alcohol Treatment Outcomes
The association between pain and alcohol treatment outcomes was assessed by extending the latent growth model of pain to predict 12-month alcohol treatment outcomes. Specifically, we tested the association between the intercept and slope parameters of the pain latent growth curve models and 12-month drinking outcomes (PDD, DDD, PHDD, MXD), while controlling for baseline levels of the outcomes. For COMBINE the 12-month outcomes represented outcomes one year following treatment (16-months following baseline assessment), whereas for UKATT the 12-month outcome represented drinking outcomes 9-months following completion of treatment (12-months following baseline assessment).
Hypothesis 2: Association between Pain and Negative Affect
The association between pain and negative affect scores over time was assessed using parallel process latent growth models. In COMBINE, we examined changes in negative latent factors across months 4 (intercept), 6.5, 12, and 16 as associated with changes in pain graded response scores across months 4 (intercept), 6.5, and 12. In UKATT we examined changes in negative latent factors across baseline to month 3 (intercept) to month 12 as associated with changes in pain graded response scores across baseline to month 3 (intercept) to month 12. In both the COMBINE and UKATT datasets we covaried the growth parameters (i.e., intercept parameters and slope parameters) across the pain and negative affect growth processes.
Hypothesis 3: Pain and Alcohol Treatment Outcomes Mediated by Negative Affect
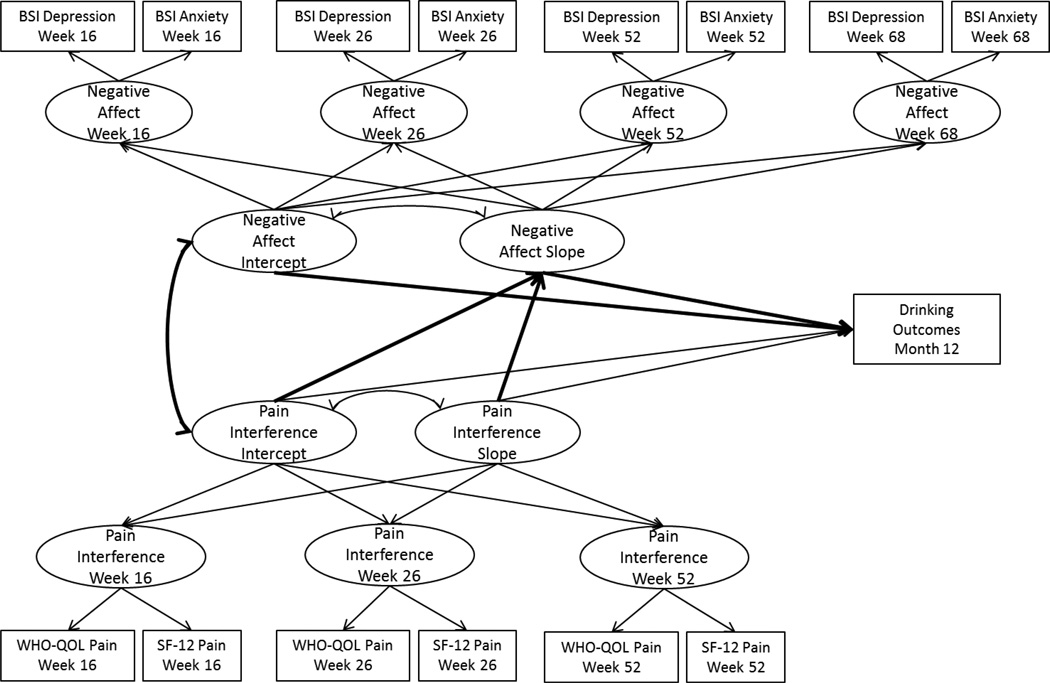
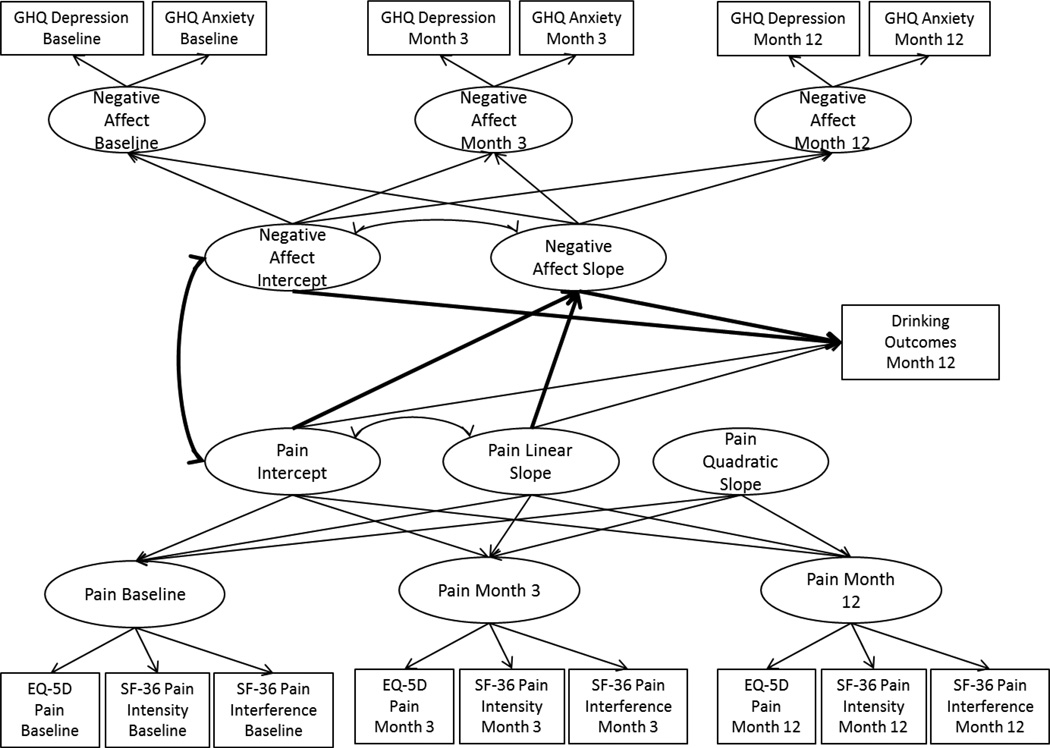
Next, we extended the parallel process growth models of pain and negative affect to include covariates and the 12-month drinking indices as outcomes of the pain and negative affect latent growth parameters, with the negative affect growth process tested as mediating the effect of the pain growth process on 12-month drinking outcomes, as shown in Figures 1a (COMBINE) and 1b (UKATT). Mediation models were estimated using the product of coefficients approach (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002), which provides an estimate of the mediated effect by multiplying two regression coefficients: (1) the regression of the mediator on the independent variable (the “a-path”), and (2) the regression of the outcome on the mediator (the “b-path”), with the independent variable included in the model (MacKinnon, 2008). The mediation models tested in the current paper examined negative affect growth (the mediator) regressed on pain growth (the independent variable), and the drinking outcomes (outcome) regressed on negative affect growth, with the inclusion of pain growth in the model. The 95% confidence intervals for the mediated effects were estimated using bootstrapping with 1000 bootstrap samples.
Figure 1a.
Parallel process growth mediation model in COMBINE. BSI = Brief Symptom Inventory; WHO-QOL = World Health Organization Quality of Life measure of pain interference; SF-12 = Short Form 12 measure of pain interference.
Figure 1b.
Parallel process growth mediation model in UKATT. GHQ = General Health Questionnaire; EQ-5D = European Quality of Life measure of pain intensity; SF-36 = Short Form 36 Health Survey measure of pain intensity and pain interference.
Exploratory Analyses: Effects of Treatment Assignment
In the final set of analyses we examined two questions regarding the effects of treatment on pain, negative affect, and alcohol treatment outcomes. First, we examined whether randomly assigned treatment condition in COMBINE (naltrexone, acamprosate, or placebo in combination with MM or CBI) or UKATT (MET or SBNT) significantly predicted pain scores by including fixed effects of treatment in the pain latent growth curve models. Second, we examined whether treatment condition moderated the association between pain, negative affect, and alcohol treatment outcomes by including moderating effects of treatment (i.e., an interaction term for centered pain scores by treatment condition) in each set of analyses described above. For both studies we created dummy-coded variables for treatment effects. In COMBINE we examined three contrasts: naltrexone (coded 1) versus no naltrexone (coded 0); acamprosate (coded 1) versus no acamprosate (coded 0); and CBI (coded 1) versus MM+CBI (coded 0). In UKATT, we examined one contrast: SBNT (coded 1) versus MET (coded 0).
Results
Descriptive Statistics
Means (standard deviations) of all continuous measures (e.g., BSI, GHQ, Form 90 drinking outcomes) and the frequency (%) of item responses on the WHO-QOL and SF-12 items (COMBINE study) and EQ-5D and SF-36 items (UKATT study) are provided in Tables 1a (COMBINE) and 1b (UKATT). In both studies, we observed reductions in pain (defined as interference in COMBINE and both pain interference and pain intensity in UKATT), depression and anxiety scores, and drinking frequency and intensity from baseline to the follow-up assessments.
Table 1a.
Descriptive Statistics, N (%) or Mean (Standard Deviation), for COMBINE Study Variables
| Baseline | 4-months | 6.5 months | 12-months | 16-months | |
|---|---|---|---|---|---|
| WHO-QOL pain interference | |||||
| 1-Not at all | 845 (62%) | -- | 705 (66%) | 608 (64%) | -- |
| 2-A little | 335 (25%) | -- | 221 (21%) | 220 (23%) | -- |
| 3-A moderate amount | 120 (9%) | -- | 95 (9%) | 90 (9%) | -- |
| 4-Very much/extremely | 51 (4%) | -- | 40 (4%) | 37 (4%) | -- |
| SF-12 pain interference | |||||
| 1-Not at all | 796 (59%) | 762 (69%) | -- | 577 (61%) | -- |
| 2-A little bit | 342 (25%) | 237 (22%) | -- | 234 (24%) | -- |
| 3-Moderately | 137 (10%) | 70 (6%) | -- | 93 (10%) | -- |
| 4-Quite a bit/extremely | 81 (6%) | 33 (3%) | -- | 47 (5%) | -- |
| BSI – Depression (range 42–80) | 61.6 (10.6) | 55.1 (10.6) | 55.5 (11.3) | 55.3 (11.0) | 55.2 (11.1) |
| BSI – Anxiety (range 38–80) | 58.4 (11.5) | 51.3 (11.1) | 51.5 (11.7) | 50.9 (11.1) | 51.1 (11.4) |
| Percent drinking days | 78.6 (22.5) | 27.3 (33.5) | 36.2 (37.8) | 38.0 (38.7) | 37.4 (39.1) |
| Drinks per drinking day | 12.9 (8.1) | 4.7 (5.8) | 5.6 (6.7) | 5.4 (6.4) | 5.3 (6.3) |
| Percent heavy drinking days | 73.7 (24.7) | 19.8 (29.9) | 27.7 (35.3) | 29.4 (36.7) | 29.3 (37.3) |
| Maximum drinks per day | 22.2 (14.0) | 6.7 (8.3) | 7.3 (8.5) | 7.1 (8.1) | 5.9 (8.1) |
| Pain scores (range −0.5 – 2.5) | 0.13 (0.73) | 0.11 (0.64) | 0.13 (0.77) | 0.15 (0.70) | -- |
| Affect scores (range −2.7 – 2.5) | 0.01 (0.94) | 0.01 (0.89) | 0.00 (0.92) | 0.01 (0.95) | 0.01 (0.95) |
Note. WHO-QOL = World Health Organization Quality of Life survey; SF-12 = Short Form Health Survey; BSI = Brief Symptom Inventory
Table 1b.
Descriptive Statistics, N (%) or Mean (Standard Deviation), for UKATT Study Variables
| Baseline | 3-months | 12-months | |
|---|---|---|---|
| EQ-5D pain intensity | |||
| 1-No pain or discomfort | 265 (36%) | 284 (44%) | 227 (40%) |
| 2-Some pain or discomfort | 419 (57%) | 332 (51%) | 293 (52%) |
| 3-Extreme pain or discomfort | 47 (7%) | 34 (5%) | 43 (8%) |
| SF-36 pain interference | |||
| 1-Not at all | 207 (28%) | 273 (42%) | 194 (35%) |
| 2-A little bit | 178 (24%) | 150 (23%) | 129 (23%0 |
| 3-Moderately | 152 (21%) | 92 (14%) | 91 (16%) |
| 4-Quite a bit | 122 (17%) | 93 (15%) | 93 (17%) |
| 5-Extremely | 72 (10%) | 39 (6%) | 54 (10%) |
| SF-36 pain intensity | |||
| 1-None | 109 (14.9%) | 169 (26%) | 137 (24%) |
| 2-Very mild | 95 (13.0%) | 105 (16%) | 83 (15%) |
| 3-Mild | 133 (18.2%) | 102 (16%) | 96 (17%) |
| 4-Moderate | 241 (32.9%) | 174 (27%) | 132 (23%) |
| 5-Severe | 110 (15.0%) | 74 (11%) | 78 (14%) |
| 6-Very severe | 44 (6.0%) | 24 (4%) | 38 (7%) |
| GHQ – Depression (range 0–21) | 7.92 (6.20) | 5.61 (6.02) | 5.24 (6.10) |
| GHQ – Anxiety (range 0–21) | 10.97 (5.38) | 8.37 (5.57) | 7.96 (5.85) |
| Percent drinking days | 78.1 (24.96) | 50.36 (38.3) | 49.59 (39.6) |
| Drinks per drinking day | 24.64 (14.73) | 14.40 (13.26) | 13.70 (13.43) |
| Percent heavy drinking days | 76.51 (25.95) | 46.78 (38.56) | 45.44 (39.91) |
| Maximum drinks per day | 35.28 (19.76) | 20.08 (17.89) | 18.95 (18.39) |
| Pain scores (range −1.49 – 2.49) | 0.06 (0.81) | −0.05 (0.82) | −0.0004 (.82) |
| Affect scores (range −1.74 – 2.79) | 0.001 (0.90) | 0.01 (0.90) | −0.01 (0.89) |
Note. EQ-5D = European Quality of Life survey; SF-36 = Short Form Health Survey; GHQ = General Health Questionnaire
Measurement and Latent Growth Models of Pain and Negative Affect
Pain graded response model
The longitudinal graded response models of pain provided an adequate fit to the observed data in the COMBINE data (χ2 (13) = 70.04, RMSEA = 0.06 (90% CI: 0.044, 0.070), CFI = 0.99) and the longitudinal graded response model of pain provided a reasonable fit to the observed data in UKATT (χ2 (43) = 279.97, RMSEA = 0.08 (90% CI: 0.077, 0.096), CFI = 0.99). Means (standard deviations) for model-generated pain scores are provided in Tables 1a and 1b.
Negative affect factor model
The longitudinal confirmatory factor analyses of the negative affect scales also provided an adequate fit to the observed data in COMBINE (χ2 (25) = 53.41, RMSEA = 0.03 (90% CI: 0.019, 0.041), CFI = 0.996) and a reasonable fit to the observed data in UKATT (χ2 (24) = 69.90, RMSEA = 0.05 (90% CI: 0.037, 0.065), CFI = 0.99). All factor loadings exceeded 0.80 in both studies. Finally, in both studies, the longitudinal confirmatory factor models were found to display strong invariance across time (item loadings and item intercepts constrained to equality). From these models we generated negative affect scores using the modal posterior estimator approach. Descriptive statistics for the negative affect scores are provided in Tables 1a (COMBINE) and 1b (UKATT).
Pain and negative affect latent growth models
The pain graded response scores and negative affect scores at each time point were then entered into separate latent growth curve models. For COMBINE, the final pain model with the intercept at month 4 and linear slope provided an excellent fit to the data (χ2 (2) = 11.23, RMSEA = 0.06 (90% CI: 0.028, 0.093), CFI = 0.99) and the final negative affect model with the intercept at month 4 and both linear and quadratic slopes provided an excellent fit to the data (χ2 (3) = 3.09, RMSEA = 0.005 (90% CI: 0.000, 0.048), CFI = 1.00). For the UKATT data, the final pain model and the final negative affect model included the intercept centered at the 3-month follow-up (end of treatment) with linear and quadratic slopes. Both models were just-identified (e.g., the model degrees of freedom equal zero) with a random linear slope and fixed quadratic slope (χ2 (0) = 0.00, RMSEA = 0.00), CFI = 1.00).
Hypotheses 1: Pain and Alcohol Treatment Outcomes
In order to test the association between pain and drinking outcomes, we examined the association between the intercept and slope parameters from the pain latent growth models and the 12-month drinking outcomes (PDD, DDD, PHDD, and MXD), while also controlling for baseline levels of the outcomes. All models provided an acceptable fit to the observed data. As shown in Table 2, results indicated that the end-of-treatment pain scores (intercepts in the latent growth models) were significantly associated with all four drinking outcomes in both COMBINE and UKATT. Thus, the hypothesis (Hypothesis 1) was supported given that pain was significantly associated with drinking outcomes. Change in pain scores over time (slope) did not significantly predict 12-month outcomes, above and beyond the intercept and baseline levels of the outcomes. Across all models, including baseline drinking as a predictor, 5–10% of the variance in 12-month drinking outcomes was explained by the model. With baseline drinking excluded from the model (results not shown), pain independently explained 1–5% of the variance in drinking outcomes.
Table 2.
Results from Analyses of Pain Growth Factors Predicting 12-Month Drinking Outcomes
| COMBINE | β | B (SE) | R2 |
|---|---|---|---|
| 12-month Percent Drinking Days (PDD) | 0.09 | ||
| Baseline PDD | 0.30 | 0.52 (0.05)** | |
| Pain interference intercept | 0.06 | 0.04 (0.02)* | |
| Pain interference slope | 0.001 | 0.01 (0.13) | |
| 12-month Drinks per Drinking Day (DDD) | 0.07 | ||
| Baseline DDD | 0.22 | 0.17 (0.02)** | |
| Pain interference intercept | 0.11 | 0.01 (0.003)** | |
| Pain interference slope | 0.01 | 0.01 (0.02) | |
| 12-month Percent Heavy Drinking Days (PHDD) | 0.06 | ||
| Baseline PHDD | 0.22 | 0.33 (0.05)** | |
| Pain interference intercept | 0.08 | 0.05 (0.02)* | |
| Pain interference slope | −0.001 | −0.006 (0.13) | |
| 12-month Maximum Drinks per Day (MXD) | 0.05 | ||
| Baseline MXD | 0.18 | 0.10 (0.02)** | |
| Pain interference intercept | 0.12 | 0.02 (0.004)** | |
| Pain interference slope | 0.01 | 0.01 (0.03) | |
| UKATT | β | B (SE) | R2 |
| 12-month Percent Drinking Days (PDD) | 0.10 | ||
| Baseline PDD | 0.25 | 0.40 (0.07)** | |
| Pain interference and intensity intercept | 0.08 | 0.04 (0.02)* | |
| Pain interference and intensity slope | 0.15 | 0.35 (0.50) | |
| 12-month Drinks per Drinking Day (DDD) | 0.16 | ||
| Baseline DDD | 0.38 | 0.34 (0.01)** | |
| Pain interference and intensity intercept | 0.09 | 0.02 (0.007)* | |
| Pain interference and intensity slope | 0.11 | 0.09 (0.14) | |
| 12-month Percent Heavy Drinking Days (PHDD) | 0.09 | ||
| Baseline PHDD | 0.25 | 0.38 (0.06)** | |
| Pain interference and intensity intercept | 0.09 | 0.05 (0.02)* | |
| Pain interference and intensity slope | 0.13 | 0.30 (0.44) | |
| 12-month Maximum Drinks per Day (MXD) | 0.15 | ||
| Baseline MXD | 0.36 | 0.33 (0.04)** | |
| Pain interference and intensity intercept | 0.10 | 0.02 (0.01)* | |
| Pain interference and intensity slope | 0.11 | 0.13 (0.24) | |
p < 0.05;
p < 0.01;
β = standardized regression coefficient; B = unstandardized regression coefficient (SE = Standard Error)
Hypothesis 2: Association between Pain and Negative Affect
The parallel process growth models of pain and negative affect scores provided a reasonable fit to the data for COMBINE (χ2 (14) = 120.08, RMSEA = 0.07 (90% CI: 0.062, 0.087), CFI = 0.989) and a slightly less than adequate fit based on RMSEA in the UKATT dataset (χ2 (6) = 63.10, RMSEA = 0.11 (90% CI: 0.08, 0.14), CFI = 0.98). In both datasets the associations between the growth processes of pain and negative affect were significant, such that the intercepts were significantly positively associated (COMBINE: B (SE) = 0.19 (0.02), p < 0.001; UKATT: B (SE) = 0.28 (0.02), p < 0.001) and the slopes were positively associated (COMBINE: B (SE) = 0.001 (0.00), p < 0.001; UKATT: B (SE) = 0.05 (0.01), p < 0.001). Thus a higher level of pain was associated with a higher level of negative affect (intercept) and increases in pain were associated with increases in negative affect (slope), providing support for Hypothesis 2. In both datasets, the intercept of pain was not significantly associated with the slope of negative affect and the intercept of negative affect was not significantly associated with the slope of pain.
Hypothesis 3: Pain and Alcohol Treatment Outcomes Mediated by Negative Affect
The parallel process growth models of pain and negative affect scores were extended to include covariates and 12-month drinking outcomes with negative affect as a mediator of the association between pain scores and drinking outcomes. All models provided an adequate fit to the data based on RMSEA and CFI. Results from the analyses were largely consistent across the COMBINE and UKATT datasets, such that in all models the negative affect growth factors (intercept and/or slope) significantly mediated the association between pain and drinking outcomes, providing support for Hypothesis 3.
In COMBINE (see Table 3), when negative affect was included in the model, the association between the pain growth factors in predicting drinking outcomes (Hypothesis 1) was no longer significant and there were significant mediation effects of both the negative affect intercept and the negative affect slope. Numerous significant covariate effects were noted across all drinking outcome models. Males and individuals with higher ADS scores had significantly higher 12-month PDD and greater readiness to change at baseline was associated with significantly lower 12-month DDD and MXD. Across all four drinking outcomes, being female, unmarried, non-Hispanic White, more severe dependence severity (as measured by ADS and DSM-IV), and reporting lower self-efficacy were significantly associated with higher negative affect (intercept) and racial minority status predicted an increase in negative affect over time (slope). Being married, unemployed, with a lower income, and more severe dependence severity were associated with greater pain (intercept). None of the covariates were significantly associated with changes in pain over time.
Table 3.
Results from Analyses of Pain Growth Factors Predicting 12-Month Drinking Outcomes Mediated by Negative Affect in COMBINE
| COMBINE | B (SE; 95% CI) | R2 | |
|---|---|---|---|
| 12-month Percent Drinking Days (PDD) (RMSEA=0.05 (90% CI: 0.04, 0.06), CFI = 0.98) | .28 | ||
| Baseline PDD | 0.48 (0.04)** | ||
| Pain interference intercept | 0.02 (0.03) | ||
| Pain interference slope | −0.71 (0.77) | ||
| Negative affect intercept | 0.10 (0.02)** | ||
| Negative affect slope | 2.89 (1.50) | ||
| Pain intercept → Affect intercept → PDD Mediation | .01 (.003; 95% CI: .01, .02)** | ||
| Pain slope → Affect slope → PDD Mediation | .97 (.71; 95% CI: 0.33, 3.23)* | ||
| 12-month Drinks/Drinking Day (DDD) (RMSEA=0.05 (90% CI: 0.04, 0.06), CFI = 0.98) | .23 | ||
| Baseline DDD | 0.14 (0.04)** | ||
| Pain interference intercept | 0.004 (0.004) | ||
| Pain interference slope | −0.07 (0.12) | ||
| Negative affect intercept | 0.02 (0.003)** | ||
| Negative affect slope | 0.40 (0.22) | ||
| Pain intercept → Affect intercept → DDD Mediation | .003 (.001; 95% CI: .002, .004)** | ||
| Pain slope → Affect slope → DDD Mediation | .13 (.10; 95% CI: .04, .45)* | ||
| 12-month % Heavy Drinking Days (PHDD) (RMSEA=0.05 (90% CI: 0.04, 0.06), CFI=.98) | .28 | ||
| Baseline PHDD | 0.33 (0.04)** | ||
| Pain interference intercept | 0.02 (0.03) | ||
| Pain interference slope | −0.69 (0.79) | ||
| Negative affect intercept | 0.11 (0.02)** | ||
| Negative affect slope | 3.19 (1.56)* | ||
| Pain intercept → Affect intercept → PHDD Mediation | .02 (.003; 95% CI: .01, .02)** | ||
| Pain slope → Affect slope → PHDD Mediation | 1.07 (.76; 95% CI: .41, 3.43)* | ||
| 12-month Maximum Drinks/ Day (MXD) (RMSEA=0.05 (90% CI: 0.04, 0.06), CFI=.98) | .23 | ||
| Baseline MXD | 0.07 (0.04)** | ||
| Pain interference intercept | 0.007 (0.01) | ||
| Pain interference slope | −0.11 (0.16) | ||
| Negative affect intercept | 0.02 (0.004)** | ||
| Negative affect slope | 0.59 (0.28)* | ||
| Pain intercept → Affect intercept → MXD Mediation | .003 (.001; 95% CI: .002, .01)** | ||
| Pain slope → Affect slope → MXD Mediation | 0.20 (.14; 95% CI: 0.07, 0.64)* | ||
p < 0.05;
p < 0.01;
B = unstandardized regression coefficient (SE = Standard Error); 95% CI = 95% confidence interval of mediated effect.
In UKATT (see Table 4), the negative affect intercept did not mediate the association between the pain intercept and DDD, PHDD, or MXD outcomes. In other words, Hypothesis 3 was not supported for the intercept of negative affect mediating the intercept of pain in predicting PDD, PHDD, and MXD. On the contrary, Hypothesis 3 was supported for the intercept of negative affect mediating the association between the pain intercept and frequency of drinking (PDD). Hypothesis 3 was also supported for the slope of negative affect. The negative affect slope (change in negative affect over time) significantly mediated the association between the pain slope (change in pain over time) and all four drinking outcomes. As with COMBINE, we found several significant covariate effects. Males, individuals with higher LDQ scores, those with lower readiness to change, and lower self-efficacy had significantly higher 12-month PDD and PHD. Greater self-efficacy at baseline and employment were also associated with significantly lower 12-month DDD and MXD. Across all four drinking outcomes, being unemployed, with more severe dependence severity (as measured by LDQ and DSM-IV), lower self-efficacy, and opiate use were significantly associated with higher negative affect (intercept) and being unemployed with greater dependence severity (measured by LDQ) predicted an increase in negative affect over time (slope). Being unemployed, greater dependence severity (measured by DSM-IV), lower self-efficacy, and opiate use were associated with greater pain (intercept). Covariates were not significantly associated with changes in pain (slope).
Table 4.
Results from Analyses of Pain Growth Factors Predicting 12-Month Drinking Outcomes Mediated by Negative Affect in UKATT
| UKATT | B (SE; 95% CI) | R2 | |
|---|---|---|---|
| 12-month % Drinking Days (PDD) (RMSEA=0.08 (90% CI: 0.06, 0.09), CFI = 0.96) | .19 | ||
| Baseline PDD | 0.30 (0.07)** | ||
| Pain interference and intensity intercept | 0.09 (0.03)* | ||
| Pain interference and intensity slope | −0.03 (0.16) | ||
| Negative affect intercept | −0.08 (0.04)* | ||
| Negative affect slope | 0.19 (0.04)** | ||
| Pain intercept → Affect intercept → PDD Mediation | −.01 (.01; 95% CI: −.03, −.001)* | ||
| Pain slope → Affect slope → PDD Mediation | .008 (.002; 95% CI: .004, .012)* | ||
| 12-month Drinks/Drinking Day (DDD) (RMSEA=0.08 (90% CI: 0.06, 0.09), CFI = 0.96) | .29 | ||
| Baseline DDD | 0.28 (0.06)** | ||
| Pain interference and intensity intercept | 0.004 (0.01) | ||
| Pain interference and intensity slope | 0.002 (0.05) | ||
| Negative affect intercept | 0.02 (0.02) | ||
| Negative affect slope | 0.06 (0.02)** | ||
| Pain intercept → Affect intercept → DDD Mediation | .004 (.003; 95% CI: −.001, .01) | ||
| Pain slope → Affect slope → DDD Mediation | .002 (.001; 95% CI: .001, .004)* | ||
| 12-month % Heavy Drinking Days (PHDD) (RMSEA=0.08 (90% CI: 0.06, 0.09), CFI= .96) | .23 | ||
| Baseline PHDD | 0.28 (0.07)** | ||
| Pain interference and intensity intercept | 0.07 (0.03)** | ||
| Pain interference and intensity slope | 0.01 (0.12) | ||
| Negative affect intercept | −0.05 (0.04) | ||
| Negative affect slope | 0.21 (0.04)** | ||
| Pain intercept → Affect intercept → PHDD Mediation | −.009 (.01; 95% CI: −.02, .004) | ||
| Pain slope → Affect slope → PHDD Mediation | .009 (.002; 95% CI: .005, .01)** | ||
| 12-month Maximum Drinks/Day (MXD) (RMSEA=0.08 (90% CI: 0.06, 0.09), CFI= .96) | .27 | ||
| Baseline MXD | 0.30 (0.05)** | ||
| Pain interference and intensity intercept | 0.005 (0.01) | ||
| Pain interference and intensity slope | 0.00 (0.07) | ||
| Negative affect intercept | 0.03 (0.02) | ||
| Negative affect slope | 0.07 (0.02)** | ||
| Pain intercept → Affect intercept → MXD Mediation | .01 (.004; 95% CI: −.002, .01) | ||
| Pain slope → Affect slope → MXD Mediation | .003 (.001; 95% CI: .001, .01)** | ||
p < 0.05;
p < 0.01;
B = unstandardized regression coefficient (SE = Standard Error); 95% CI = 95% confidence interval of mediated effect.
Effects of Treatment Assignment
Results from conditional latent growth curve models indicated that none of the treatment contrasts in either COMBINE or UKATT had a significant effect on the intercept or slope of pain scores over time. Treatment condition also did not moderate the associations between pain and drinking outcomes or associations between pain and negative affect in COMBINE (all p > 0.10).
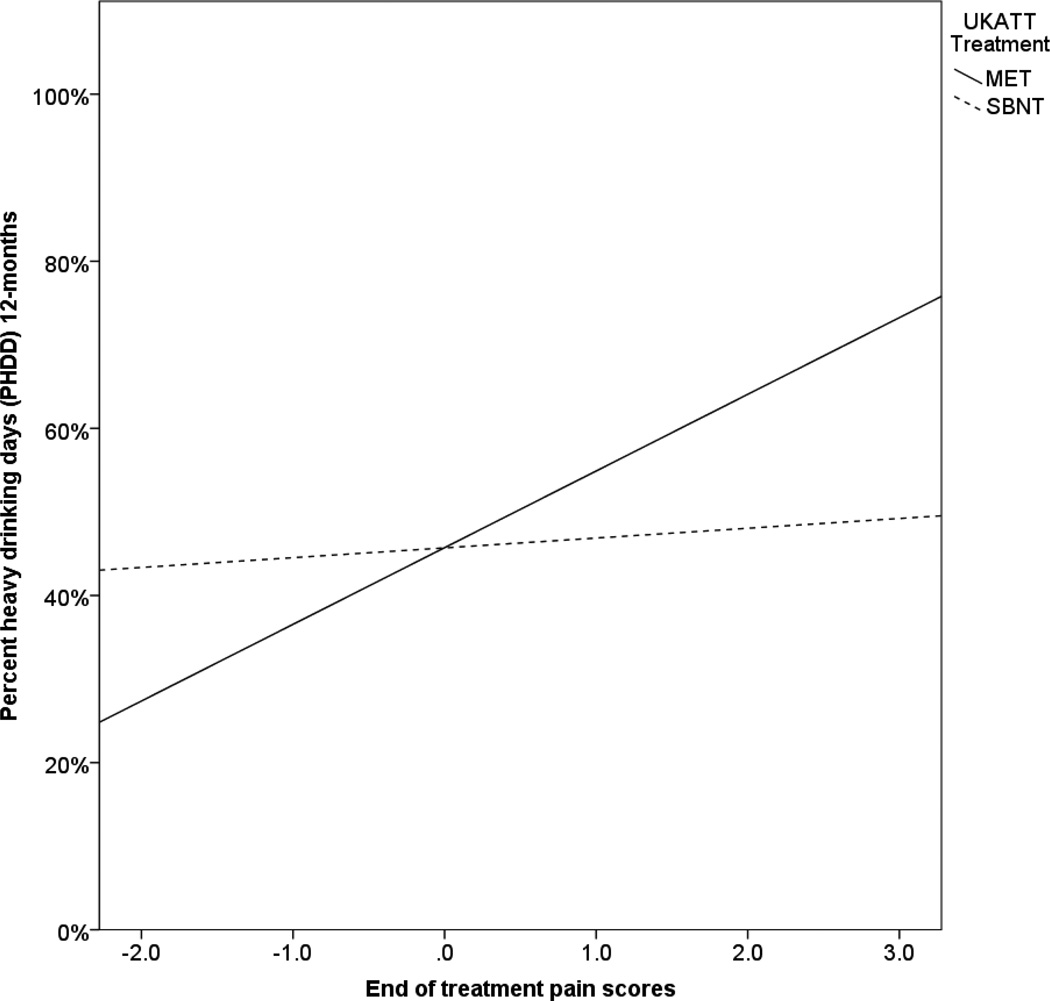
In UKATT, treatment significantly moderated the association between the pain intercept (end of treatment pain scores) and percent heavy drinking days (PHDD) at the 12-month follow-up (B (SE) = −0.01 (0.004), p = 0.045). As shown in Figure 2, the association between the pain intercept and 12-month PHDD was significant for the MET condition (B (SE) = .14 (.04), p < 0.001), but was non-significant for the SBNT condition (B (SE) = .007 (0.04), p = 0.87). Treatment also significantly moderated the association between the pain intercept and the negative affect intercept (B (SE) = −0.01(0.006), p = 0.028); however, the simple slope analysis revealed that pain and negative affect were significantly positively associated in both treatment conditions, with a stronger association in MET (standardized β = 0.46), as compared to SBNT (standardized β = 0.37).
Figure 2.
Association between pain scores at the end of treatment and percent heavy drinking days by treatment condition in UKATT. MET = Motivation enhancement therapy; SBNT = Social behavior and network therapy
Results from the moderated mediation analyses were also significantly different by treatment condition in UKATT for the DDD outcome. The mediating effect of the negative affect slope was only significant in the association between pain slopes and DDD for the MET condition (indirect effect B (SE) = 0.002 (0.001), 95% CI: 0.001, 0.004), while the mediating effects were not significant for the SBNT condition (indirect effect B (SE) = .002 (0.002), 95% CI: −0.001, 0.004). Treatment condition moderated the effect of the “b-path” (i.e., negative affect slope in predicting DDD), with the association between negative affect slope and DDD significantly positive in the MET condition and non-significant in the SBNT condition. Thus, the SBNT intervention attenuated the mediating effect of negative affect on DDD in UKATT.
Discussion
The findings from current study suggest that pain and negative affect are closely associated among individuals who received treatment for AUD and that negative affect significantly mediated the association between pain and drinking outcomes. This suggests that negative affect-mediated pain may be an important risk factor in the alcohol relapse process. These results support all three hypotheses regarding the associations between pain, negative affect, and drinking outcomes. Specifically, the level of pain at the end of treatment (i.e., pain intercept) significantly predicted drinking frequency and intensity at 12-months post treatment (COMBINE) and 9-months post treatment (UKATT), supporting the first hypothesis that pain and drinking outcomes would be significantly associated. Interestingly, the slopes of the pain growth factors were not significantly associated with drinking outcomes, after controlling for baseline drinking outcomes and pain intercepts. Thus, it may be most important to assess the level of pain at the end of treatment and changes in pain following treatment may be less predictive of ultimate drinking outcomes. The results also indicated that pain and negative affect were significantly associated (supporting Hypothesis 2) and that negative affect significantly mediated the association between pain and drinking outcomes (supporting Hypothesis 3).
The finding that negative affect significantly mediated the association between pain and drinking outcomes is consistent with a growing literature on the importance of negative affect as a mediator of drinking outcomes (Allan, Albanese, Norr, Zvolensky, & Schmidt, 2015; Black et al., 2012; Kelly, Hoeppner, Stout, & Pagano, 2012). Targeting negative affect in the treatment of AUDs has been described previously (Lowman, Allen, & Stout, 1996; Witkiewitz & Villarroel, 2009), and the current results would suggest that interventions to reduce negative affect might be particularly important among individuals with chronic pain. The results from the current study are also consistent with a growing literature on the association between pain and addiction treatment outcomes (Brennan, Schutte, SooHoo, & Moos, 2011; Caldeiro et al., 2008; Sheu et al., 2008). To our knowledge, this is the first study to examine the association between pain and alcohol treatment outcomes as mediated by negative affect. Moreover, the results were largely replicated across two different randomized clinical trials conducted in two different countries (US and UK) that included patients at very different levels of physical health severity and disparate drinking rates at baseline (as seen in Tables 1a and 1b).
The differential findings by treatment conditions in UKATT, but not COMBINE, were particularly intriguing and deserve further exploration and replication. In UKATT, the associations between pain and drinking outcomes, pain and negative affect, and the full mediational model were moderated by treatment condition. The SBNT condition attenuated the associations, such that greater pain did not predict significantly greater PHDD and greater pain did not predict as strong of an association with negative affect, as compared to the MET condition. COMBINE included a comparison of medications (naltrexone, acamprosate, or placebo) to one behavioral intervention (CBI), whereas UKATT compared a lower intensity behavioral intervention (MET) with a higher intensity behavioral intervention (SBNT). Thus, it could be the intensity of the behavioral intervention or the content of the SBNT intervention was important for attenuating the associations between pain, negative affect, and drinking outcomes. Given the fairly well established efficacy of behavioral interventions for persistent pain (e.g., Ehde, Dillworth, & Turner, 2014; Gatchel, Peng, Peters, Fuchs, & Turk, 2007), a possibility is that the SBNT helpfully altered participants’ responses to their pain experiences, as well as in relation to alcohol use.
SBNT also attenuated the association between changes in negative affect and DDD, suggesting that individuals who experienced greater negative affect did not show corresponding increases in DDD at the 12-month follow-up. Previous research has found behavioral treatments for addiction, including cognitive behavioral treatment, may be partially effective by changing individual responses to negative affect such that individuals tend not to drink or use drugs in response to negative affect (Hunter, Witkiewitz, Watkins, Paddock, & Hepner, 2012). The SBNT intervention of UKATT utilized cognitive and behavioral strategies to build social support networks that were supportive of reduced drinking or abstinence and it is possible that increasing social support also provided an avenue for attenuating the reaction of frequent heavy drinking in response to both pain and negative affect. Numerous studies have found social support networks to be important in the recovery process following treatment for AUD (e.g., Havassy, Hall, & Wasserman, 1991; Timko, Finney, & Moos, 2005; Walter et al., 2006). Consistent with these correlational studies, a recent experimental study found that social partners reduced alcohol relapse-like behavior in prairie voles (Hostetler & Ryabinin, 2014). Research on chronic pain has also found social support to predict better pain outcomes in correlational studies (Montoya, Larbig, Braun, Preissl, & Birbaumer, 2004) and experimental animal studies (Gabriel, Marcus, Honig, & Joosten, 2010; Vachon et al., 2013). In addition, there is research to suggest that individuals with chronic pain who indicate satisfaction with social support show decreased levels of depressed mood and pain intensity (Lopez-Martinez, Esteve-Zarazaga, & Rameriz-Maestre, 2008) as well as pain interference (Kerns, Rosenberg, & Otis, 2002). Future research should be conducted to examine whether SBNT may be an effective treatment for comorbid pain and AUD.
Strengths and Limitations
The strengths of the current study include the replication of findings across two large datasets and the careful statistical approach to both datasets. Statistical procedures included the replication of longitudinal latent variable models to study the associations between constructs over time, while also controlling for multiple covariates and handling missing data using maximum likelihood estimation (Hallgren & Witkiewitz, 2013). The fact that the two clinical trials represent different samples, with regard to severity and nationality, is an additional strength. Further, the findings were replicated using different measures of pain and negative affect in each of the datasets.
The limitations include the lack of a measure of pain intensity in COMBINE and the lack of a multidimensional pain measure in either study. The COMBINE study assessed pain interference only and UKATT assessed both pain intensity and interference. We elected to combine these into a single latent factor, simply labeled as “pain” in the present analyses, partially because the pattern of findings across the two studies was similar and partially due to the practical fact that no other measures were available to us. However, variability in responses to pain intensity and pain interference may have influenced the pattern of results in some way, and future studies in this area would benefit from more uniform and thorough assessment of both pain intensity and interference. The use of single measures for negative affect in each dataset is also a limitation. Finally, the current study was limited by looking at drinking outcomes only at a single follow-up time point (12-months post-treatment in COMBINE, 12-months post-baseline in UKATT), rather than examining time-varying associations between pain, negative affect, and drinking outcomes.
Conclusions and Future Directions
This study was the first to identify a longitudinal association between indicators of pain , negative affect, and drinking outcomes following AUD treatment. Chronic pain and AUD are both chronic conditions that often require years of maintenance therapies. It is critical to gain a better understanding of the association between pain and AUD treatment outcomes, including potential mechanisms of the association (e.g., negative affect). Studying the associations between pain, negative affect, and AUD treatment outcomes at self-report, behavioral, and neurobiological levels may also be critical for the development of behavioral treatment options for individuals with comorbid pain and AUD. The correlational nature of the longitudinal panel data used in the current study prevents any inferences regarding the directionality of the associations between pain, negative affect, and alcohol use; however, future research employing ecological momentary assessment design could potentially disentangle temporal ordering of the associations in real-time.
The current study also holds numerous implications for intervention research. Targeting negative affect in treatment might be particularly useful among individuals with comorbid chronic pain and AUD. There has been a growing focus on the utility of mindfulness-based interventions in the treatment of chronic pain and separately in the treatment of AUD and other substance use disorders (Garland et al., 2014; Bowen et al., 2014), with some studies finding that mindfulness-based interventions may be particularly beneficial for individuals with comorbid mood disorders (Witkiewitz & Bowen, 2010; Zautra et al., 2008). Future treatment research may also consider the findings from the current study with regard to the attenuation of the pain-negative affect-drinking associations via the social behavior and network therapy utilized in UKATT.
Public Health Significance.
This study highlights the important associations between physical pain interference and intensity, negative affect, and alcohol use following treatment for an alcohol use disorder. Social network behavior therapy, which is one type of behavioral intervention for alcohol use disorders, may be particularly useful for preventing alcohol use in response to physical pain and negative affect.
Acknowledgments
This research was supported by a National Institute on Alcohol Abuse and Alcoholism Grant 1R01 AA022328 (to Katie Witkiewitz), a Senior Career Scientist Research and Mentoring Award 2K05 AA16928 (to Stephen A. Maisto, principal investigator), and by a training fellowship to Megan Kirouac (1T32 AA0018108-01A1; Barbara S. McCrady, principal investigator).
References
- Allan NP, Albanese BJ, Norr AM, Zvolensky MJ, Schmidt NB. Effects of anxiety sensitivity on alcohol problems: evaluating chained mediation through generalized anxiety, depression and drinking motives. Addiction. 2015;110:260–268. doi: 10.1111/add.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, & Association, A. P. Diagnostic and statistical manual of mental disorders. 4th ed. Arlington, VA, US: American Psychiatric Publishing, Inc; 1995. xv–223. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2320703. [DOI] [PubMed] [Google Scholar]
- Black JJ, Tran GQ, Goldsmith AA, Thompson RD, Smith JP, Welge JA. Alcohol expectancies and social self-efficacy as mediators of differential intervention outcomes for college hazardous drinkers with social anxiety. Addictive Behaviors. 2012;37:248–255. doi: 10.1016/j.addbeh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Booker EA, Haig AJ, Geisser ME, Yamakawa K. Alcohol use self-report in chronic back pain- relationships to psychosocial factors, function performance and medication use. Disability Rehabilitation. 2003;25:1271–1277. doi: 10.1080/09638280310001608609. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry. 2014;71:547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, Moos RH. Painful medical conditions and alcohol use: a prospective study among older adults. Pain Medicine. 2011;12:1049–1059. doi: 10.1111/j.1526-4637.2011.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Byrne BM, Shavelson RJ, Muthén BO. Testing for the equivalence of factor covariance and mean structures: The issue of partial measurement invariance. Psychological Bulletin. 1989;105:456–466. [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, Baer JS, Nichol P, Kivlahan DR, Saxon AJ. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction. 2008;103:1996–2005. doi: 10.1111/j.1360-0443.2008.02358.x. [DOI] [PubMed] [Google Scholar]
- Chow S-M, Witkiewitz K, Grasman R, Hutton RS, Maisto . A regime-switching longitudinal model of alcohol lapse-relapse. In: Molenaar PCM, Lerner RM, Newell KM, editors. Handbook of Developmental Systems Theory and Methodology. New York, NY: Guilford Press; 2013. pp. 397–424. [Google Scholar]
- COMBINE Study Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcoholism: Clinical and Experimental Research. 2003;27:1107–1122. doi: 10.1097/00000374-200307000-00011. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12878917. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Donovan DM. Conceptualizations of relapse: A summary of psychological and psychobiological models. Addiction. 1996;91:S5–S13. [PubMed] [Google Scholar]
- Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. Journal of Personality. 2004;72:1133–1159. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory: An introductory report. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 1983;13:595–605. [PubMed] [Google Scholar]
- DiClemente CC, Carbonari JP, Montgomery RP, Hughes SO. The Alcohol Abstinence Self-Efficacy scale. Journal of Studies on Alcohol. 1994;55:141–148. doi: 10.15288/jsa.1994.55.141. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8189734. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Hughes SO. Stages of change profiles in outpatient alcoholism treatment. Journal of Substance Abuse. 1990;2(2):217–35. doi: 10.1016/s0899-3289(05)80057-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2136111. [DOI] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist. 2014;69:153–166. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- European Quality of Life Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10109801. [DOI] [PubMed] [Google Scholar]
- Gabriel AF, Marcus MAE, Honig WMM, Joosten EAJ. Preoperative housing in an enriched environment significantly reduces the duration of post-operative pain in a rat model of knee inflammation. Neuroscience Letters. 2010;469:219–223. doi: 10.1016/j.neulet.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Garland EL, Franken IHA, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2012;222:17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology. 2014;82:448–459. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Goldberg DP. The detection of psychiatric illness by questionnaire. Oxford: Oxford University Press; 1972. [Google Scholar]
- Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcoholism: Clinical and Experimental Research. 2013;37:2152–2160. doi: 10.1111/acer.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havassy BE, Hall SM, Wasserman DA. Social support and relapse: commonalities among alcoholics, opiate users, and cigarette smokers. Addictive Behaviors. 1991;16:235–246. doi: 10.1016/0306-4603(91)90016-b. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1663695. [DOI] [PubMed] [Google Scholar]
- Heather N, Honekopp J. A revised edition of the Readiness to Change Questionnaire. Addiction Research & Theory. 2008;16:421–433. [Google Scholar]
- Hostetler CM, Ryabinin AE. Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology. 2014;39:152–157. doi: 10.1016/j.psyneuen.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hunter SB, Witkiewitz K, Watkins KE, Paddock SM, Hepner KA. The moderating effects of group cognitive-behavioral therapy for depression among substance users. Psychology of Addictive Behaviors. 2012;26:906–916. doi: 10.1037/a0028158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RD, Rosenberg R, Otis JD. Self-appraised problem solving and pain-relevant social support as predictors of the experience of chronic pain. Annals of Behavioral Medicine. 2002;24:100–105. doi: 10.1207/S15324796ABM2402_06. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Hoeppner B, Stout RL, Pagano M. Determining the relative importance of the mechanisms of behavior change within Alcoholics Anonymous: a multiple mediator analysis. Addiction. 2012;107:289–299. doi: 10.1111/j.1360-0443.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9311924. [DOI] [PubMed] [Google Scholar]
- Little TD. Longitudinal structural equation modeling. New York: 2013. [Google Scholar]
- Little TD, Preacher KJ, Selig JP, Card NA. New developments in latent variable panel analyses of longitudinal data. International Journal of Behavioral Development. 2007;31:357–365. [Google Scholar]
- Lopez-Martinez AE, Esteve-Zarazaga R, Rameriz-Maestre C. Perceived social support and coping responses are independent variables explaining pain adjustment among chronic pain patients. Journal of Pain. 2008;9:373–379. doi: 10.1016/j.jpain.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Lowman C, Allen J, Stout RL. Replication and extension of Marlatt’s taxonomy of relapse precipitants: overview of procedures and results. The Relapse Research Group. Addiction. 1996;91(Suppl):S51–S71. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8997781. [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. New York: Taylor & Francis Group; 2008. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR. Form 90: A structured assessment interview for drinking and related behaviors. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 1996. [Google Scholar]
- Montoya P, Larbig W, Braun C, Preissl H, Birbaumer N. Influence of social support and emotional context on pain processing and magnetic brain responses in fibromyalgia. Arthritis and Rheumatism. 2004;50:4035–4044. doi: 10.1002/art.20660. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus users guide (Version 7) Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C. Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction. 1994;89:563–572. doi: 10.1111/j.1360-0443.1994.tb03332.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8044122. [DOI] [PubMed] [Google Scholar]
- Ruta D, Garratt A, Abdalla M, Buckingham K, Russell I. The SF 36 health survey questionnaire. A valid measure of health status. BMJ. 1993;307:448–449. doi: 10.1136/bmj.307.6901.448-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima F. Estimation of latent ability using a response pattern of graded scores. Psychometrika Monograph Supplment. 1969;34 [Google Scholar]
- Samejima F. Graded response model. In: van der Linden WJ, Hambleton R, editors. Handbook of Modern Item Response Theory. New York, NY: Springer-Verlag; 1997. [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12090408. [PubMed] [Google Scholar]
- Sheu R, Lussier D, Rosenblum A, Fong C, Portenoy J, Joseph H, Portenoy RK. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Medicine. 2008;9:911–917. doi: 10.1111/j.1526-4637.2008.00420.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. In: Conceptual issues in the study of relapse. Gossop M, editor. New York, NY, US: Travistock/Routledge; 1989. pp. 149–179. [Google Scholar]
- Skinner HA, Horn JL. Alcohol Dependence Scale (ADS) user’s guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- Sutton SR. Interpreting relapse curves. Journal of Consulting and Clinical Psychology. 1979;47:96–98. doi: 10.1037//0022-006x.47.1.96. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/429669. [DOI] [PubMed] [Google Scholar]
- Timko C, Finney JW, Moos RH. The 8-year course of alcohol abuse: gender differences in social context and coping. Alcoholism: Clinical and Experimental Research. 2005;29(4):612–21. doi: 10.1097/01.alc.0000158832.07705.22. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15834227. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug and Alcohol Dependence. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14687956. [DOI] [PubMed] [Google Scholar]
- UKATT Research Team. United Kingdom Alcohol Treatment Trial (UKATT): Hypotheses, design and methods. Alcohol and Alcoholism. 2001;36:11–21. doi: 10.1093/alcalc/36.1.11. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11139410. [DOI] [PubMed] [Google Scholar]
- UKATT Research Team. Effectiveness of treatment for alcohol problems: findings of the randomised UK alcohol treatment trial (UKATT) BMJ. 2005;331:541. doi: 10.1136/bmj.331.7516.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon P, Millecamps M, Low L, Thompsosn SJ, Pailleux F, Beaudry F, Stone LS. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behavioral and Brain Functions. 2013;9:22. doi: 10.1186/1744-9081-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Duersteler-MacFarland KM, Weijers H-G, Boening J, Wiesbeck GA. Social factors but not stress-coping styles predict relapse in detoxified alcoholics. Neuropsychobiology. 2006;54:100–106. doi: 10.1159/000096991. [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8628042. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K. Predictors of heavy drinking during and following treatment. Psychology of Addictive Behaviors. 2011;25:426–438. doi: 10.1037/a0022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology. 2010;78:362–374. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Villarroel NA. Dynamic association between negative affect and alcohol lapses following alcohol treatment. Journal of Consulting and Clinical Psychology. 2009;77:633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA. Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE Study and the United Kingdom Alcohol Treatment Trial. Addiction. doi: 10.1111/add.12964. in press. Advance online publication http://dx.doi.org/10.1111/add.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow KM, Eltherington LG. Feeling no pain: alcohol as an analgesic. Pain. 1988;32:159–163. doi: 10.1016/0304-3959(88)90064-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3362554. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World Health Organization Quality of Life. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Nicassario P, Tennen H, Finan P, Irwin MR. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(3):408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]