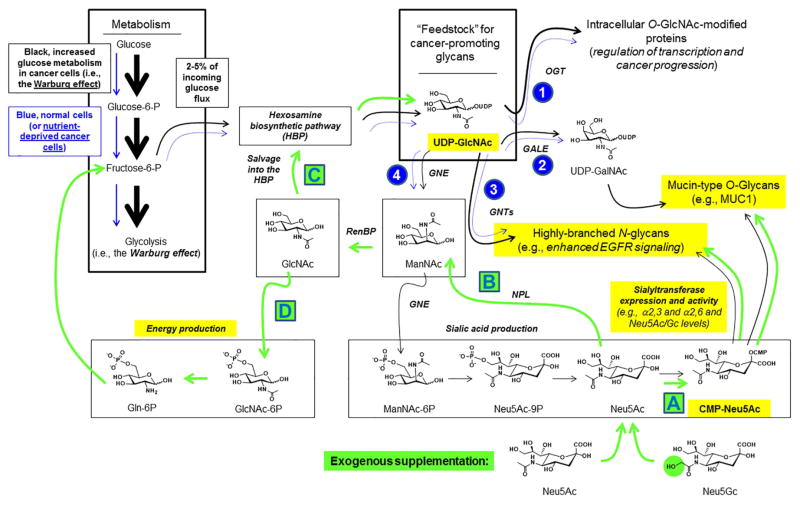
Figure 1. Monosaccharide metabolism and supplementation strategies.
Cancer cells are characterized by the “Warburg effect” wherein the cells use greatly increased levels of glucose (indicated by the black arrows throughout the diagram). One consequence of increased glucose utilization by cancer cells is increased production of UDP-GlcNAc, which promotes cancer progression by several complementary glycan-based mechanisms including (1) O-GlcNAc protein modification, (2) production of mucin-type O-glycans such as those that decorate MUC1, (3) activation of GlcNAc transferases 4 and 5, which produce highly-branched N-glycans linked to cancer including through promotion of EGFR signaling, and (4) sialic acid biosynthesis via ManNAc production. In all of these cases, lower glucose uptake found naturally in normal cells or induced by nutrient deprivation in cancer cells is expected to reduce UDP-GlcNAc levels and attenuate these cancer-promoting mechanisms (as indicated schematically by the smaller blue arrows). Supplementation of nutrient-deprived cells with sialic acids (e.g., either Neu5Ac or Neu5Gc, bottom) leads to several plausible outcomes that can counteract the effects of nutrient deprivation and maintain the production of cancer-promoting glycans (indicated with the green arrows) including (A) direct incorporation into cellular glycans, (B) catabolism to ManNAc, which can be re-used for sialic acid production (not shown) or conversion to GlcNAc. In turn, GlcNAc can (C) be salvaged by the HBP and used to replenish cellular supplies of UDP-GlcNAc or (D) be routed into a pathway that creates fructose-6-phosphate that can be used for energy production. Specific endpoints investigated in this report are highlighted in yellow.