Abstract
AIM: This paper describes the procedure of detection of Helicobacter pylori (H. pylori ) in bile specimens in patients suffering frombenign diseases of biliary ducts (lithiasis with/without nonspecific cholangitis).
METHODS: The group of 72 patients entering the study consisted of 32 male and 40 female (45% and 55%, respectively). Bile was obtained during ERCP in 68 patients, and during cholecystectomy in 4 patients. A fast urease test (FUT) to determine the existence of H. pylori in gastric mucosa was carried out for all the patients during the endoscopic examination. The existence of genetic material of H. pylori was determined by detection of ureA gene by the method of nested PCR. The results of this reaction were shown by electrophoresis on 10 g•L⁻¹ agarose gel in a band of 256 bp.
RESULTS: The majority of the patients included in our study had biliary lithiasis without signs of cholangitis (48 patients, 67%), whereas other patients were complicated by cholangitis (17 patients, 24%). Seven patients (9%) had normal ERCP, forming thus the control group. In the group of patients with lithiasis 26 patients (54.2%) had positive PCR of H. pylori in bile and among the patients with associated cholangitis positive PCR was detected in 9 patients (52.9%). Among the seven patients with normal ERCP only one (14%) had positive PCR of H. pylori. A high percentage of H. pylori infection of gastric mucosa was observed (57 patients, 79%). It was also observed that its slightly higher positivity was in the patients with distinct bile pathology: 81% FUT positive patients in the group with choledocholithiasis alone and 76% in the group with choledocholithiasis associated with cholangitis. Seventy-one percent of the patients with regular findings had positive FUT.
CONCLUSION: The prevalence of H. pylori infection both in bile and in gastric mucosa in patients with benign diseases of biliary ducts does not show a statistically significant difference in relation to the prevalence of the same with the patients with normal ERCP. The existence of H. pylori infection possibly does not play a role in pathogenesis of benign biliary diseases.
INTRODUCTION
Helicobacter pylori (H. pylori) is a microaerophilic and Gram-negative microorganism which could represent the main causative agent in the development of chronic antral gastritis, duodenal ulcer, or even gastric cancer[1-3]. In the last few years, the interests of scientists in the correlation between H. pylori infection and various extradigestive diseases[4] have been significantly increasing. Due to the complexity of pathophysiologic mechanisms of the infection with this microorganism, diagnostic procedures for the detection of H. pylori infection in extragastric specimens did not meet high standards. Fast urease test for the detection of H. pylori infection in stomach often had false negative or false postive results[5]. Microscopic analysis of the specimens by means of various staining also proved to be nonspecific without sufficient sensitivity to detect this organism. The incubation process takes even from 3 to 7 d in order to obtain cultures of H. pylori. This was the reason to undertake cloning of urease gene of H. pylori , which was specific for this species, thus obtaining high sensitivity and specificity[6]. Urease genes (EMBL acc. No. X17079)[7] were sequestrected and specific H. pylori oligonucleotid primers were synthesized. Having used fumes of these oligonucleotid primers for detection and identification of H. pylori , the method of PCR (polymerase chain reaction) permits the recognition of H. pylori even in a trace, by multiplying the aimed genetic sequence of a specific ureA gene[8].
This paper describes the procedure of H. pylori microorganism detection in specimens of bile with the patients subject to the test suffering from benign diseases of biliary ducts (lithiasis with/without nonspecific cholangitis).
MATERIALS AND METHODS
The study was carried out on 72 patients, admitted at the Institute for Digestive Diseases (the Clinic for Gastroenterology and Hepathology and First Surgical Clinic) of the University Clinical Centre of Serbia in Belgrade. Laboratory workup was accomplished at the Gastrointestinal Research Laboratory, University of Rostock. The group of 72 patients who were tested consisted of 32 male and 40 female (45% and 55, respectively). The age range was from 11-90 yrs. The average age of all the patients was 56.7 years with SD ± 16.45. Thirty two patients (44%) had undergone previous cholecystectomy.
The patients were examined at the University Clinical Centre of Serbia from September 20, 1998 to October 5, 1999. Prior to this, the patients did not undergo any endoscopic biliary theraputical procedures. In 68 patients, the specimens of bile were obtained during endoscopic retrograde cholangio-pancreatography (ERCP) and in 4 patients during cholecystoctomy. In all the patients which underwent ERCP, endoscopic papillotomy (EPT) was carried out with a variety of subsequent extraction procedures (basket, mechanical lithotripsy, balloon). Sampling of bile in those cases was performed prior to EPT. The existence of H. pylori in gastric mucosa was checked by fast urease test in all the patients at the Clinic of Gastroenterology and Hepathology of the Clinical Centre of Serbia during endoscopic examination.
DNA was isolated from bile specimens by a sequence of procedures including respectively: centrifugation, followed by a 2-hour incubation in lysis buffer, extraction via phenol chloroform, precipitation with acid ethanol, and dissolution in Tris-EDTA buffer. The final DNA sample was tested in respect of the existence of ure A gene by means of PCR method. In order to detect H. pylori infection of bile ducts nested (two-step) PCR was used, with two pairs of primers: outer primer with its own sense and antisense as follows: 5'-GCCAATGGTAAATTAGTTCC-3' (s) 5'-TTACTCCTTAATTGTTTTTAC-3' (as) and the other pair is so-called inner primer with its own sense and antisense: 5'-TTCTTTGAAGTGAATAGATGC-3' (s) 5'-ATAGTTGTCATCGCTTTTAGC-3' (as)[9]. Taq polymerase, 5MU•L⁻¹ (Perkin Elmer Biosystems) was used during the reaction. The master mix for the first reaction (outer primers) consisted of 5 μL of DNA template, 3 μL of MgCl2, 5 μL Taq reaction buffer II, 4 μL dNTPs, 0.2 μL Taq polymerase, 1.3 μL of outer sense primer, 1.2 μL of outer antisense primer, and H2O up to the final volume of 50 μL. The master mix for the second reaction (inner primers) differed from the first one in the amount of primers: 1.7 μL of outer sense primer, 1.0 μL of outer antisense primer. Both PCR reactions were performed in a DNA thermal cycler in three steps, 1 min. at 94 °C, 1 min. at 50 °C, and 1 min. at 72 °C, for 25 cycles each. The H. pylori gene was presented by electrophoresis on 10 g•L⁻¹ agarose gel at the level of 258 base pairs.
RESULTS
Based on the clinical tests, we diagnosed biliary lithiasis in the patients included in our study. Forty-eight of them (67%) had no signs of cholangitis (Figure 1), and 17 cases were complicated by cholangitis (24%, Figure 2). Seven patients (9%) had normal ERCP findings and constituted the control group.
Figure 1.
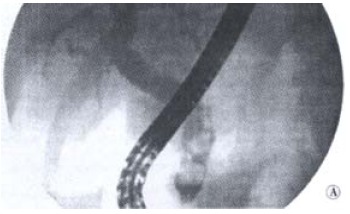
ERCP: Biliary calculosis
Figure 2.
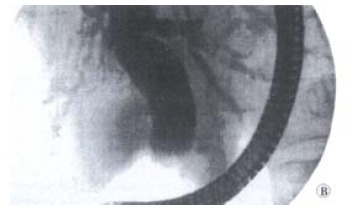
ERCP: Cholangitis
In the group of patients with lithiasis 54.2% patients had a positive PCR H. pylori in bile and in the group of patients with inflammation a positive PCR was detected in 52.9% of the patients (Table 1). Among the seven patients with regular findings only one patient (14%) was PCR positive. There was a statistically significant difference in the positivity of PCR H. pylori in the joint group of patients who had non-malignant diseases of bile ducts (gallstones and inflammation) in relation to the controls (Chi-square test, P = 0.0467). After the tested group was diivided into two basic pathologies and the statistical analysis repeated, it was clear that only the patients with lithiasis alone had a significantly higher frequency of positive H. pylori in bile (P = 0.0486), contrary to the ones with associated cholangitis (Fischer's precision test, P = 0.0967).
Table 1.
H. pylori positivity in bile (PCR) and in gastric mucosa (FUT)
| Test |
Biliary lithiasis |
controls | |
| without cholangitis | with cholangitis | ||
| PCR + | 26 | 9 | 1 |
| PCR - | 22 | 8 | 6 |
| FUT + | 39 | 13 | 5 |
| FUT - | 9 | 4 | 2 |
PCR: for ureA gene; FUT: Fast urease test
Fast urease test revealed a high percentage of H. pylori of gastric mucosa (57 patients - 79%). There was a slightly higher positivity in patients with distinct biliary pathology - 81% FUT positive patients in the group with cholelithiasis and 76% in the group with associated cholangitis, compared to the controls, where FUT was positive in 71%. There was no statistical difference between the groups (χ² test, P = 0.5957).
Finally, if we compare findings of H. pylori in bile (by PCR method) with the same in gastric mucosa (by FUT method), we come to the conclusion that there is no statistically significant difference between them. However, if we extract only the subgroup of patients with lithiasis then the difference becomes highly significant (χ², P = 0.0045).
DISCUSSION
Bile acids in physiological concentrations inhibit the growth of various sorts of intestinal bacteria[10], including lactobacilli and clostridia, which are sensitive to unconjugated bile acids, such as cholic, deoxycholic and lithocholic acids. Since H. pylori is a Gram negative bacteria, it also shows sensitivity in vitro to deoxycholic and chenodeoxycholic acids[11]. They are the main free bile acids in human bile. However, under different pathological conditions, this inhibition factor of H. pylori growth can be changed: for example, the concentration of various matter in bile can be influenced by biliary obstruction[12]. Further, the in vivo inhibitory effect of bile acids to H. pylori has been proven by various studies through testing the role of H. pylori in biliary reflux gastritis, which, however, has given contradictory results[13], as some studies suggested that biliary reflux from the duodenum into the antrum did not affect the growth of H. pylori in antrum.
In our study, out of the total of 72 patients H. pylori was identified in bile of 36 patients (50%). This study demonstrates that there is a possibility to identify this microorganism by means of PCR method in the environment which is known to inhibit its growth under in vitro conditions. Since H. pylori infection was more frequent in the patients with the diseases of biliary ducts than in the controls (53.8% vs 14.3%), it is possible to suppose that under the pathological conditions there was a change of the conditions for the growth of this bacteria in vivo and this would be the subject of further research. Although it seems unlikely that H. pylori could grow in the environment which contains bile, it has been proven for some of Helicobacter species to be living in the gallbladder (H. hepaticus, H. bilis, H. pullorum and others)[14]. Fox et al[15] detected H. bilis, H. pullorum and H. rappini by means of PCR method in 23 patients with the diagnosis of chronic cholecystitis although there were no cases where microorganisms had grown from bile cultures. Based on these data, it is possible that H. pylori caused certain idiopathic hepatobiliary diseases. H. pullorum is known to cause infection in people and chicken manifested with diarrhoea or increased hepathic enzymes and liver enlargement. H. rappini, which causes abortion in sheep and acute insufficiency of liver of the sheep fetus, was isolated in people complaining of diarrhoea. Finally, H. bilis was proved to be a cause of hepatitis in mice[16].
Offner et al[17] announced in 1994 that the presence of Mr130 proteins caused crystalization of cholesterol. It was also found that CagA protein of H. pylori had this identical molecular weight, and also that both of these proteins had crossed reactions with human leucin-aminopeptidase[18]. Figura et al[19] found anti-CagA antibodies in 15 of 16 bile specimens in patients undergoing cholecystoctomy for stones and were proven to have Cag A H. pylori within the stomach. All these findings support the role of these microorganisms in the initiation of crystalization of cholesterol which induces cholelithiasis. Recent researches devoted to the studies of Helicobacter sp. In different diseases of biliary ducts and liver show that Helicobacter can survive not only in stomach but also in human bile, and, additionally, that it can be the cause of various hepatobiliary diseases. For this reason, Roe et al[20] tested the survival of Helicobacter sp. In bile of the patients having various diseases of biliary system which contained changed bile acids or bile acids to which H. pylori was resistant. The study included 20 patients with intrahepatic lithiasis, three patients had pancreas cancer and two patients had common bile duct cancer. Bile was obtained by means of PTBD method and tested in respect of the presence of 16 s rRNA specific gene of H. pylori by PCR method. The result of the PCR were the product of 375 bp. After the sequencing and analysis of sequences, 20 (80%) of PCR product were suited to H. pylori genome while the rest of 5 (20%) were suited to H. bilis genome. Based on the obtained results the authors concluded that H. pylori was the most important and the most frequent cause of infection among all sorts of Helicobacter sp. in diseases of biliary ducts. However, the authors did not give any explanation how the infection of biliary ducts with this bacteria happens but suggested further research in future.
In order to analyse the path and source of infection of biliary ducts utilizing the most senzitive methodology, we tested the positivity of H. pylori by PCR method (in bile) and FUT (in gastric mucosa) the result of which was a statistically very significant difference. This surprising finding could imply that the pathogenesis of biliary system and gastric mucosa are independent. In this difference the main portion belongs to lithiasis, since in the case of the subgroup with associated cholangitis no statistically significant difference was found. Taking into account that in the subgroup of lithiasis there were some deviations in the positivity of H. pylori towards the controls, it is clear that biliary pathogenesis is not uniform in all its forms. It has been pointed out earlier that H. sp. exist in the biliary system and gallbladder of the patients belonging to the Chilean population[13], being one of the main causes of chronic inflammation which can stimulate forming of biliary stones. Ponzetto et al[21] tested the presence of H. sp. in bile and mucosa of bile bladder of the patients having cholelithiasis and prevalence of H. pylori antibodies in serum of the patients. Sixty four patients were subjected to cholecystectomy and from whom bile was collected at operation as well as the mucosa of gallbladder. The specimens of serum were compared to the specimens of 610 blood donors. Serum was tested in respect of the presence of specific IgG antibodies in relation to H. pylori (ELISA). Bile and mucosa of bile bladder were tested by means of PCR method with respect to the presence of genetic sequence 16 s rRNA H. sp. In 22 out of 64 tested specimens of bile PCR for H. sp. was positive. H. pylori infection was significantly more frequent in the patients with cholelithiasis than in the controls, and H. pylori DNA was also present in the bile of the patients.
Our clinical and molecular biological tests for H. pylori in patients with biliary diseases were undertaken in a larger population than that previously reported.
It is possible to conclude that PCR method can detect H. pylori infection of biliary ducts. Prevalence of H. pylori infection in the patients with benign diseases of bile ducts does not show a statistically significant difference in relation to the prevalence with the patients presentign with normal ERCP. However, in the patients with biliary lithiasis there is a certain difference which is on the borderline of statistical significance. The analysis of H. pylori in gastric mucosa by means of FUT method did not have a statistically significant difference either among the patients with benign biliary pathology and controls. Based on all the data stated above it can be said that the presence of H. pylori infection, either in bile or in gastric mucosa, does play a role in pathogenesis of benign biliary diseases although more explicit conclusions require a larger control group.
AKNOWLEDGEMENT
Warmest gratitude to the Department of Medicine, University of Rostock, and particularly to the Head and staff of the Gastrointestinal Research Laboratory for their kind help, support and contribution in this article.
Footnotes
Edited by Pan BR and Zhang JZ
Supported by Gastrointestinal Research Laboratory, Department of Medicine, University of Rostock, Germany
References
- 1.Hobsley M, Tovey FI. Helicobacter pylori: the primary cause of duodenal ulceration or a secondary infection. World J Gastroenterol. 2001;7:149–151. doi: 10.3748/wjg.v7.i2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Läuter J, Labenz J, Leodolter A, et al. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study) World J Gastroenterol. 2001;7:243–247. doi: 10.3748/wjg.v7.i2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: A systematic overview. CMAJ. 1994;150:177–185. [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarrini A, Franceschi F, Gasbarrini G, Pola P. Extraintestinal pathology associated with Helicobacter infection. Eur J Gastroenterol Hepatol. 1997;9:231–233. doi: 10.1097/00042737-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Hazell SL, Borody TJ, Gal A, Lee A. Campylobacter pyloridis gastritis I: Detection of urease as a marker of bacterial colonization and gastritis. Am J Gastroenterol. 1987;82:292–296. [PubMed] [Google Scholar]
- 6.Clayton CL, Wren BW, Mullany P, Topping A, Tabaqchali S. Molecular cloning and expression of Campylobacter pylori species-specific antigens in Escherichia coli K-12. Infect immun. 1989;57:623–629. doi: 10.1128/iai.57.2.623-629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton CL, Pallen MJ, Kleanthous H, Wren BW, Tabaqchali S. Nucleotide sequence of two genes from Helicobacter pylori encoding for urease subunits. Nucleic Acids Res. 1990;18:362. doi: 10.1093/nar/18.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin TT, Yeh CT, Wu CS, Liaw YF. Detection and partial sequence analysis of Helicobacter pylori DNA in the bile samples. Dig Dis Sci. 1995;40:2214–2219. doi: 10.1007/BF02209009. [DOI] [PubMed] [Google Scholar]
- 10.Floch MH, Gershengoren W, Elliott S, Spiro HM. Bile acid inhibition of the intestinal microflora--a function for simple bile acids. Gastroenterology. 1971;61:228–233. [PubMed] [Google Scholar]
- 11.Hänninen ML. Sensitivity of Helicobacter pylori to different bile salts. Eur J Clin Microbiol Infect Dis. 1991;10:515–518. doi: 10.1007/BF01963941. [DOI] [PubMed] [Google Scholar]
- 12.Xu GR, Kirk CJ, Goode AW. Changes in biliary lipid concentrations in bile duct obstruction: An experimental study. J R. Soc Med. 1986;79:522–527. doi: 10.1177/014107688607900908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellosalo J, Alavaikko M, Laitinen S. Effect of biliary tract procedures on duodenogastric reflux and the gastric mucosa. Scand J Gastroenterol. 1991;26:1272–1278. doi: 10.3109/00365529108998624. [DOI] [PubMed] [Google Scholar]
- 14.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 15.Stanley J, Linton D, Burnens AP, Dewhirst FE, On SL, Porter A, Owen RJ, Costas M. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology. 1994;140(Pt 12):3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 16.Franklin C, Riley L, Hunziker R. Enteropathic lesions in scid mice infected with Helicobacter bilis. Lab Anim Sci. 1997;47:438–439. [Google Scholar]
- 17.Offner GD, Gong D, Afdhal NH. Identification of a 130-kilodalton human biliary concanavalin A binding protein as aminopeptidase N. Gastroenterology. 1994;106:755–762. doi: 10.1016/0016-5085(94)90712-9. [DOI] [PubMed] [Google Scholar]
- 18.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figura N, Cetta F, Angelico M, Montalto G, Cetta D, Pacenti L, Vindigni C, Vaira D, Festuccia F, De Santis A, et al. Most Helicobacter pylori-infected patients have specific antibodies, and some also have H. pylori antigens and genomic material in bile: is it a risk factor for gallstone formation. Dig Dis Sci. 1998;43:854–862. doi: 10.1023/a:1018838719590. [DOI] [PubMed] [Google Scholar]
- 20.Roe IH, Kim JT, Lee HS, Lee JH. Detection of Helicobacter DNA in bile from bile duct diseases. J Korean Med Sci. 1999;14:182–186. doi: 10.3346/jkms.1999.14.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponzetto A, Vergnano G, Soldati T, Cutufia MA, Giustetto A, Angelino R, Pellicano R, Leone N, Arena V, Rizzetto M, et al. Detection of Helicobacter pylori in the bile of patients with cholelithiasis. Gut. 1999;45:A162. [Google Scholar]


