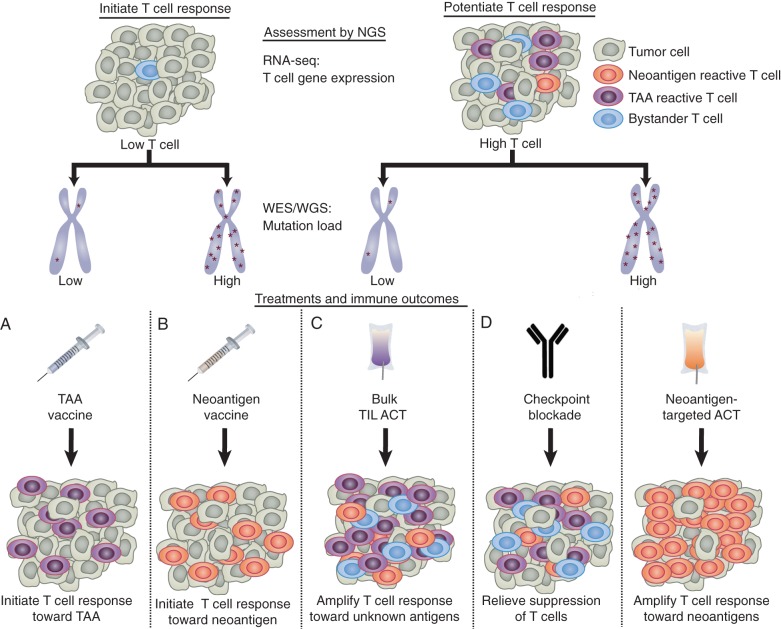
Figure 1.
Proposed use of NGS data to personalize immunotherapy. The goals of immunotherapy are to either potentiate pre-existing antitumor T-cell responses or initiate antitumor T-cell responses if antitumor immunity is low. The level of pre-existing tumor immunity can be assessed by measuring the level of T-cell markers in RNA-seq data [84]. The total mutation load can be determined using whole-genome or whole-exome sequencing (WGS/WES). (A) Patients with low T cells and low mutation load may benefit most from personalized tumor-associated antigen (TAA)-specific vaccines to activate T cells towards highly expressed TAA, which can be identified using RNA-seq data. (B) Patients with low T cells and a high mutation load may benefit most from neoantigen-specific vaccines. (C) Patients with high T cells but a low mutation load may benefit most from standard ACT, to amplify intratumoral T cells against undefined antigens. (D) Patients with high T cells and high mutation load may benefit most from either checkpoint blockade, to relieve T-cell suppression, or neoantigen-targeted ACT, to amplify the mutation-reactive T-cell response. Combination therapies can also be used.