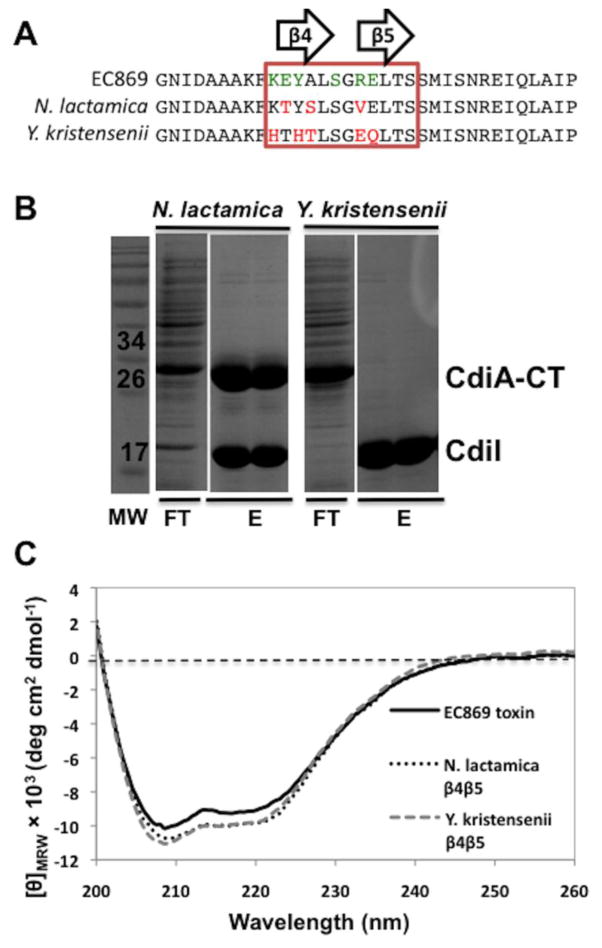
Fig. 7.
Toxin β-hairpin sequence contributes to complex binding affinity. (a) Protein sequence alignment of the β-hairpin region (boxed) of CdiA-CTo11EC869, CdiA-CTNlact and CdiA-CTYkris. CdiA-CTo11EC869 residues that interact with CdiIo11EC869 are shown in green. Red residues indicate sequence differences with respect to CdiA-CTo11EC869. (b) CdiIo11 EC869-His6 was co-expressed with CdiA-CTo11EC869 containing the β-hairpins from either N. lactamica or Y. kristensenii then purified by Ni2+-affinity chromatography and analyzed by SDS-PAGE. The molecular mass standards are indicated in kilodaltons (kDa). The flow-though (FT) and wash fractions (W1 and W2) are indicated followed by elution with imidazole gradient. (c) CD spectra of purifiedCdiA-CTo11EC869/Nlact and CdiA-CTo11 EC869/Ykris show similar secondary-structure content compared to wild-type CdiA-CTo11EC869.