Abstract
Danshen has been widely used in the treatment of cardiovascular diseases while Danshensu [3(3,4dihydroxyphenyl) 2 hydroxy propanoic acid, DSS], a major water-soluble component of Danshen has also been explored as an important compound in Danshen. In the present study, DSS was tested in isolated rat hearts of ischemia reperfusion (I/R) model to investigate its cardioprotective activity and explore the potential molecular mechanism against oxidative stress. The isolated rat hearts were used to perform global ischemia for 30 min, followed by 30 min reperfusion. DSS significantly decreased the level of the marker enzymes (creatine kinase and lactate dehydrogenase) from the coronary effluents and myocardial infarction size. This could markedly contribute to the recovery of cardiac function after I/R injury. DSS also had ROS scavenging activity and boosted endogenous antioxidants such as SOD, CAT, MDA, GSH-PX and HO-1 activities by activating nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway which was mediated by Akt and ERK1/2 in western blot analysis. Our results demonstrated a cardioprotective effect of DSS on isolated heart against oxidative stress during I/R injury. This mechanism might be related to the enhancement of antioxidant defense system by activating Akt/ERK1/2/Nrf2 signaling pathways. This work could provide experimental evidence in treating cardiovascular disease by the use of traditional Chinese medicine particularly in myocardial ischemia reperfusion injury.
Keywords: Danshensu, myocardial ischemia reperfusion injury, oxidative stress, Akt/ERK1/2/Nrf2 signaling pathway
Introduction
Since the initial description of ischemia reperfusion (I/R) injury by Jennings and his colleagues in 1960 [1], the study of reperfusion injury has become of significance to various studies done on cerebrovascular, hepatic, renal and cardiovascular systems. Ischemia and reperfusion occurs in various situations, including thrombolysis, percutaneous coronary angioplasty, organ transplantation and coronary bypass [2]. Prompt reperfusion is still the most effective way of reducing heart injury in acute myocardial infarction but reperfusion has also the potential risk of worsening tissue damage thereafter ischemia it usually accelerates the deterioration of cardiac function [3-5]. Reactive Oxygen Species (ROS) generation, intracellular calcium overload, ATP depletion, myocardial apoptosis and endothelial dysfunction are all considered as the end result of I/R cascade [6,7].
Oscar Langendorff first designed the apparatus in 1895 for the purpose of studies conducted on the isolated mammalian heart. Consequently, models of isolated hearts have been used to study ischemia reperfusion injury research [8-10]. Ischemia reperfusion injury research, this could provide the basic knowledge in studying fundamentals of cardiac physiology including heart function, coronary flow and the entire cardiac metabolic activity [11].
Salvia miltiorrhiza (Danshen), a famous Chinese herb, has been widely used in both Asia and western countries in treating microcirculatory disorders like cerebrovascular, cardiovascular, renal inactivity and hepatic dysfunction. Danshen is well recognized for its cardiovascular activity. Its water soluble constituent, Danshensu (DSS) mimics the parent herb in managing cardiovascular disorders. Previously, studies revealed that DSS could improve circulation in the smaller arteries, reduce ROS generation [12-14], inhibit cardiofibroblast proliferation and improve collagen synthesis [15-17], inhibit cell apoptosis [14,18,19] and protect heart against ischemia reperfusion injury [20-22]. DSS could also prevent myocardial hypertrophy [23,24] and atherosclerosis [25,26] .
Myocardial damage induced by ischemia-reperfusion is due, at least in part, to the generation of ROS. These oxygen species are highly reactive and can quickly overwhelm the cell’s endogenous free radical scavenging system. Several studies have shown that the addition of antioxidants or ROS scavengers delay the onset or attenuate ischemia-reperfusion injury [27-29]. Nuclear factor erythroid-2-related factor 2 (Nrf2) is an important transcription factor that regulates the antioxidant response [30,31]. Activation of the Nrf2 pathway affords protection against oxidative stress during I/R [32,33].
In the current work, therefore, Danshensu was tested in rat isolated heart ischemia/reperfusion injured model to investigate its cardioprotective effect and explore whether it can inhibit oxidative stress through Nrf2 signaling.
Materials and methods
Materials
Danshenshu (98%, purity) was purchased from Chinese Institute for Drug and Biological Product Control (Beijing, China). The kits for determination of creatine kinase (CK), lactate dehydrogenase (LDH), superoxide dismutase (SOD), glutathione peroxides (GSH-PX), and catalase (CAT), malondialdehyde (MDA) was obtained from Jiancheng Bioengineering Institute (Nanjing, China). 2, 3, 5-triphenyl tetrazolium chloride (TTC) was purchased from Solarbio (Beijing, China). 2’, 7’-dichlorouoresce in diacetate (H2DCFDA) was from invtrogen (USA). BCA Protein Assay Kit was from Thermo Scientific (USA). Transcriptor First Strand cDNA Synthesis Kit and FastStart Universal SYBR Green Master Mix Kit were obtained from Roche (Switzerland). phospho-Akt (Ser473) (#4060), total Akt (#4691), phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) (#4370), p44/42 MAPK (ERK1/2) (#4695), GAPDH (#5174) were purchased from Cell Signaling Technology.Nrf2 (C-20) (sc-722) and goat anti-rabbit secondary IgG-HRP (sc-2004) were purchased from Santa Cruz Biotechonlogy, Inc.
Animal and ethics
Male Sprague Dawley rats (300 ± 20 g) were purchased from Beijing Vital River Lab Animal Technology Co. Ltd. Ambient temperature (22 ± 2°C) and relative humidity (60 ± 5%) were maintained. The rats were allowed to acclimatize to the housing facilities before the experiments and eat a standard diet and drink ad libitum. This study was carried out in accordance with the recommendations in the Guidance for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of China and the protocol approved by the Laboratory Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (Permit Number: TCM-LAEC2014004).
Isolated rat heart perfusion and Langendorff procedure
Administration of anaesthesia, operation, medication and heart organ harvest summerized the whole protocol in this experiment. Rats were anesthetized with sodium pentobarbital (50 mg/kg) injected intraperitoneal. A thoracotomy was then performed and hearts were rapidly excised into an ice-cold heparinized (5 U/mL) modified buffered Krebs-Henseleit solution (KHs). After removal of lungs and surrounding tissues, the aorta was attached to the perfusion device where hearts were perused at a constant flow retrograde, according to the method of Langendorff with a non-recirculated Krebs-Henseleit solution of the following composition (mM): NaCl 118; KCl 4.7; CaCl2 1.25; MgS04 1.2; KH2P04 1.2; glucose 11.0; NaHC03 25.0 gassed with carbogen (95% O2 and 5% CO2) at 37° to yield a physiological pH of 7.4. The hearts were perfused with constant pressure of approximately 65 mmHg and left for 20 minutes to permit recovery of function and stabilize the rhythm The left ventricular end diastolic pressure was maintained at 5~10 mmHg and coronary flow (CF) was measured by collecting the effluent dripping from the heart perfusate. Cardiac functions were evaluated upon left ventricular developed pressure (left ventricle end systolic pressure minus left ventricle end diastolic pressure; LVDP = LVSP-LVEDP), the recovery rate of maximal and minimum rate of pressure development (± dp/dt max, %) at the end of reperfusion period, and heart rate (HR). LVDP, ± dp/dt max and HR were calculated from the left ventricular pressure curve. These parameters were recorded continuously on a computer using Powerlab data acquisition system (A.D. Instruments, Castle Hill, Australia/8SP Chart 5 software) [34].
Subsequently, hearts were randomly assigned to the following groups: 1) Control group (hearts perfused with KH buffer for 90 min); 2) I/R group (hearts that were allowed to stabilize for 20 min prior to being subjected to 30 min global ischemia achieved by discontinued KHs perfusion, followed by 30 min reperfusion); 3) DSS 1 µM group (hearts perfused with DSS 1 µM for 10 min after 20 min of stabilization, global ischemia was performed for 30 min, followed by 30 min reperfusion); 4) DSS 10 µM group (hearts perfused with DSS 10 µM for 10 min after 20 min of stabilization, global ischemia was performed for 30 min, followed by 30 min reperfusion) as in Figure 1.
Figure 1.
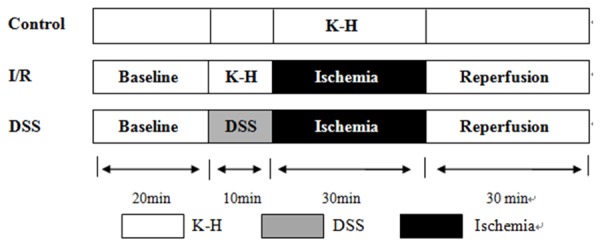
Langendorff protocol. Baseline: 20 minutes stabilization with KH buffer. Drug treatments: the heart was perfused with 1 μM, 10 μM DSS for 10 min before ischemia. Ischemia/reperfusion: 30 min global ischemia achieved by breaking off KHs perfusion, followed by 30 min reperfusion.
Creatine kinase and lactate dehydrogenase assay
As additional markers of myocardial injury, CK and LDH were determined from the coronary effluent which was collected before ischemia, at 5, 10, 20 and 30 min of reperfusion, as previously described [35]. And they were spectrophotometrically assayed by using commercial kits. CK and LDH levels were expressed in units per liter of coronary effluents.
Determination of myocardial infarction size
After 30 min reperfusion, hearts were removed from Langendorff apparatus, aortic root excised and kept in -20°C for 30 min. Frozen hearts were cut transversely into different slices like 2-3 mm which were incubated in 1% 2, 3, 5-triphenyl tetrazolium chloride (TTC) dissolved in 0.1 M phosphate buffer (pH 7.4) at 37°C for 15 min. The infracted areas were displayed as the area unstained by TTC [36]. The extents of the necrosed areas were quantified by two independent and blind observers. Total area of infarct and risk areas were then calculated and expressed as a percentage of the whole heart area. The slices were then photographed for further analysis.
Assessment of reactive oxygen species (ROS) production
At the end of reperfusion, heart homogenates for ROS analysis were prepared in ice-cold saline and the obtained supernatant centrifuged at 3000 rpm for 15 min then assayed using a BCA protein assay kit standardized to BSA. ROS production was determined by oxidation of 2’, 7’-dichlorouoresce in diacetate (CMDCF-DA) (invtrogen Lot NO. 1220683). The heart homogenates in each group were incubated with 0.5 μ mol/L DCFH-DA at 37°C for 30 min. The fluorescence was detected by EnSpire Multimode Plate Reader (PerkinElmer, USA) excited at 488 nm and the emitted at 525 nm.
Determination of MDA, SOD and GSH-PX activity in isolated heart homogenates
Heart homogenates for SOD, MDA and GSH-PX assay were done as previously described and the supernatant obtained after centrifugation at 3000 rpm for 15 min. MDA, SOD and GSH-PX activity in these samples were determined using commercially available kits by EnSpire Multimode Plate Reader (PerkinElmer, USA) at different absorbance.
Real time quantitative RT-PCR analysis
After 30 min reperfusion period, total RNA was isolated from left ventricular myocardial tissue by using Trizol reagent according to the standard protocol. Total RNA was reverse transcribed in 20 μl of a reaction mixture that contained Reverse Transcriptase, Reaction Buffer of 5× concentration, RNase inhibitor, DNTP mix, Random Hexamer Primer, using Transcriptor First Strand cDNA Synthesis Kit (Roche), run at 25°C for 10 min, 50°C for 1 h, 85°C for 5 min and stored at 4°C. Quantification of gene expression was performed with real-time quantitative RT-PCR machine (Real-Time PCR System, Bio-Rad, USA), using Fast Start Universal SYBR Green Master Mix Kit (Roche) and the specific primers (Table 1). The 25 μl total volumes in PCR consisted of specific primer (0.2 μM final concentrations), 50 ng of CNDA template, 2× SYBR Green master mix and water, PCR-grade. Amplification was performed under the following cycle conditions: 10 min at 95°C, 40 cycles of 15 s denaturation at 95°C, and annealing at 60°C for 40 s. The threshold cycle (CT) value was determined and the relative mRNA expression of the genes was calculated as follows: 2-ΔΔCT with ΔΔCT = CTGAPDH-CT gene of interest. Negative control the reaction mixture was run without cDNA. And analysis carried out in triplicates.
Table 1.
Primer Sequences for Cu/Zn SOD, CAT, HO-1, Nrf2 and GAPDH for RT-PCR analysis
| Primers | Sequences (5’-3’) | |
|---|---|---|
| Cu/Zn SOD | Forward | AGATGACTTGGGCAAAGGTG |
| Reverse | CAATCCCAATCACACCACAA | |
| CAT | Forward | ACATGGTCTGGGACTTCTGG |
| Reverse | CCATTCGCATTAACCAGCTT | |
| HO-1 | Forward | CACGCATATACCCGCTACCT |
| Reverse | CCAGAGTGTTCATTCGAGCA | |
| Nrf2 | Forward | TTCCTCTGCTGCCATTAGTCAGTC |
| Reverse | GCTCTTCCATTTCCGAGTCACTG | |
| GAPDH | Forward | ATGATTCTACCCACGGCAAG |
| Reverse | CTGGAAGATGGTGATGGGTT | |
Western blotting analysis
After 30 min global ischemia and 10 min reperfusion, heart great vessels and atria were trimmed away and the ventricles were cut open, weighed, and then snap-frozen and stored at -80°C awaiting analysis. Proteins were extracted with ice-cold lysis buffer and centrifuged at 12000× g, 10 min, and the resultant supernatant assayed using BCA protein assay kit standardized to BSA. Equal amounts of total protein (40 µg) were loaded and then separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane. The membranes were then blocked in 5% TBST fat free milk (blocking buffer) for 2 h, shortly washed and incubated overnight at 4°C with specific primary antibodies against Akt, p-Akt (Ser473) (1:1000 dilution), ERK 1/2, p-ERK 1/2 (Thr202/Tyr204) (1:1000 dilution) and Nrf2 (C-20) (1:500 dilution). Anti-rabbit secondary antibody (1:10000 dilution) was then used and incubated for 2 h. Proteins were detected using a chemiluminescence (ECL) system, according to the manufacturer’s instructions. Band intensities were quantified using Imaging System Analysis software (VersaDoc Mp5000, BIO-RAD). Relevant band intensities were quantified after normalization to the amount of GAPDH protein as positive control.
Results
Effect of DSS on cardiac function in Langendorff isolated rat hearts
The analysis of hemodynamic parameters on cardiac function was as in Figure 2. At the baseline, there was no significant difference observed in the coronary flow (CF), heart rate (HR) and left ventricular developed pressure (LVDP). Compared to control, CF at 5, 10, 20 and 30 min reperfusion and HR, LVDP and the recovery rate of ± dp/dt max at the end of reperfusion period were decreased significantly in the I/R group. The results also showed that the recovery of cardiac function in I/R was augmented by DSS pretreatment, as evidenced by the significant increase of CF, HR, LVDP and the recovery of ± dp/dt max. These results suggested that DSS could contribute to the recovery of cardiac function after ischemia and reperfusion and increase the tolerance of hearts against I/R injury in a concentration-dependent manner.
Figure 2.
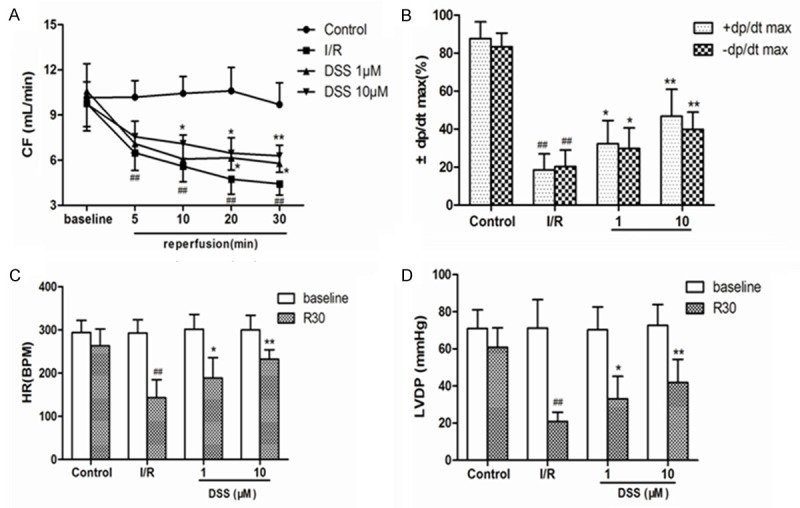
Effects of DSS on cardiac functional parameters in isolated rat hearts. The hearts underwent 20 min of stabilization, 30 min of ischemia and 30 min of reperfusion, drugs were administered before ischemia for 10 min. A. Coronary flow (CF); B. The recovery rate of maximal and minimum rate of pressure development (± dp/dt max, %) at the end of reperfusion period; C. Heart rate (HR) at the stabilization and the end of reperfusion; D. Left ventricular developed pressure (LVDP) at the stabilization and the end of reperfusion. Data expressed as mean ± SD, n = 8. #P < 0.05, ##P < 0.01 versus Control group. *P < 0.05, **P < 0.01 versus I/R group.
Protective effect of DSS on myocardial infarct size and CK, LDH release
The infarct size of hearts in the DSS 1 µM and 10 μM groups averaged to 34.90 ± 9.15% and 24.00 ± 6.35%, respectively, which reduced significantly in the I/R group (43.29 ± 5.86%) (Figure 4). It demonstrated that DSS could have reduced the percentage of the infarct area. CK and LDH in coronary effluent during the baseline and after 5, 10, 20 and 30 min of reperfusion in the four experimental groups were as shown in Figure 3. There was no significant difference in the baseline values of CK and LDH in the four groups. It was obvious that the levels of CK and LDH levels significantly increased in early stage of reperfusion and reached the peak in 20 min reperfusion. In contrast to I/R group, pretreatment with DSS at 10 μM for 10 min before ischemia significantly decreased LDH and CK release in the previous four reperfusion times. These results confirmed that DSS protected the heart from ischemia reperfusion injury.
Figure 4.
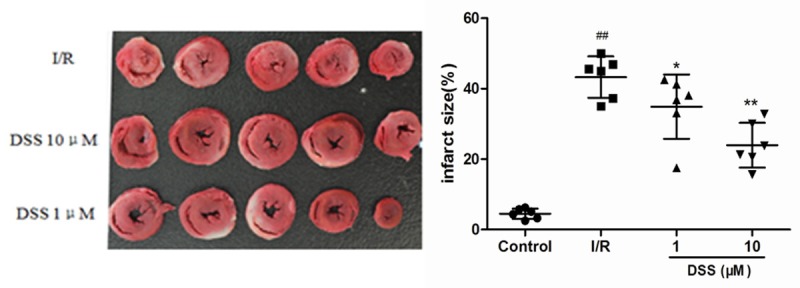
Effects of DSS on myocardial infarction size. Infarct size was evaluated by triphenyltetrazolium chloride staining following 30 min global ischemia and 30 min reperfusion in isolated rat hearts model. Data expressed as mean ± SD, n = 6. #P < 0.05, ## P < 0.01 versus Control group. **P < 0.01 versus I/R group.
Figure 3.
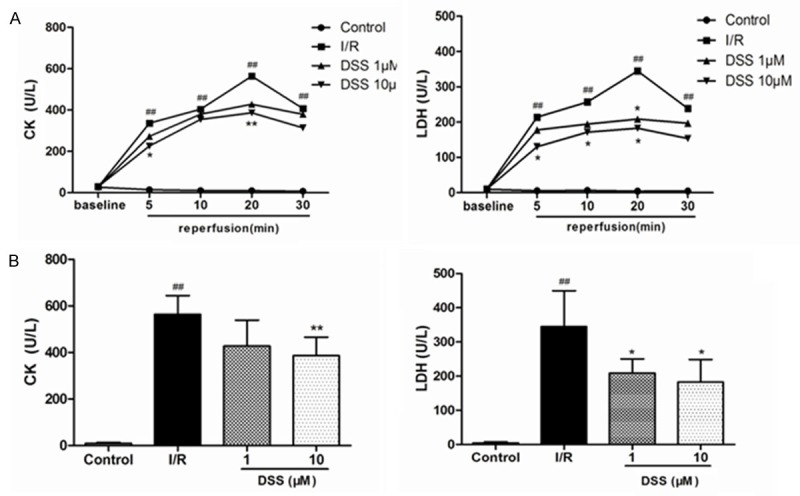
Effects of DSS on the level of marker enzymes from the coronary effluents. A. Effects of DSS on CK and LDH release in the coronary effluent of isolated rat hearts following ischemia and reperfusion (I/R). B. CK and LDH release in the coronary effluent at 20 min reperfusion. Data expressed as mean ± SD, n = 8. #P < 0.05, ##P < 0.01 versus Control group. *P < 0.05, **P < 0.01 versus I/R group.
DSS mediates cardioprotection against oxidative stress
Reactive Oxygen Species are the major contributors to I/R injury and its overproduction was found to be deleterious to the heart. Many studies have shown that the treatment of antioxidants or ROS scavengers could attenuate I/R injury. Therefore, we evaluated whether DSS mediated cardioprotection could be partially attributed to a decrease in oxidative stress mediated by ROS generation. Ischemia/reperfusion induced a significant increase in ROS in I/R group, which reflected by increased DCFDA fluorescence (Figure 5A). This effect was greatly attenuated by pretreatment of DSS.
Figure 5.
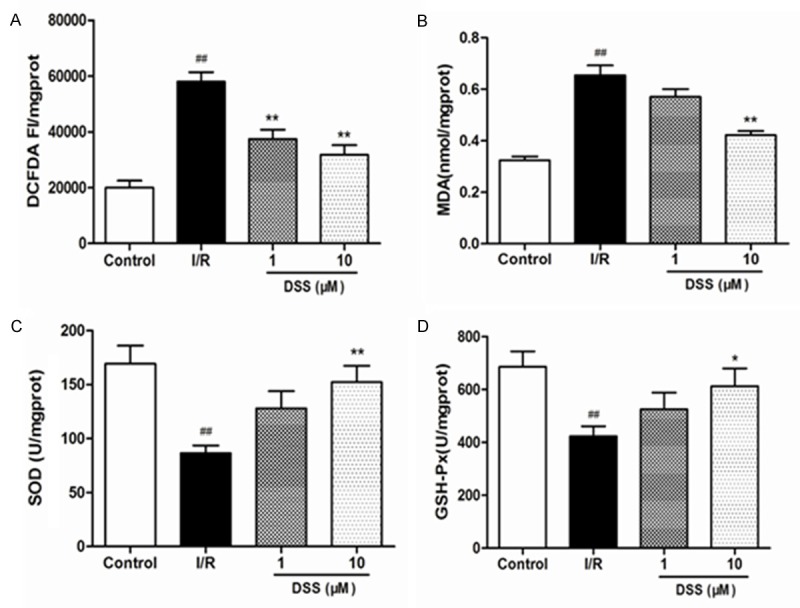
Effects of DSS on the contents of ROS, MDA, SOD, and GSH activity after 30 min reperfusion in isolated hearts homogenate. A. The content of ROS; B. The content of MDA; C. The activity of SOD; D. The activity of GSHPX. Data expressed as mean ± SD, n = 6. #P < 0.05, ##P < 0.01 versus Control group. **P < 0.01 versus I/R group.
Under normal circumstances, basal generation of ROS efficiently detoxified by endogenous enzymatic antioxidants such as SOD, GSH-Px and CAT. However, during ischemia/reperfusion injury, excess ROS generation exceeds the capacity of endogenous oxidant defense mechanisms hence leading to deleterious ROS-mediated oxidative stress injury. We therefore proposed that DSS affected activity of the antioxidant enzymes by increasing the levels of SOD, GSH-Px and decreasing the level of MDA. Compared to control group, MDA levels in I/R group was markedly increased, while SOD and GSH-PX levels greatly reduced. This was a sign that oxidative stress took place. After pretreatment with 10µM DSS, MDA levels were greatly lowered in comparison to I/R group, while SOD and GSK-PX had a great activity (Figure 5B-D). Subsequently RT-PCR was done to quantify the expression of Cu/Zn SOD, CAT, HO-1 and Nrf2 mRNA expression DSS pretreated isolated rat hearts. DSS at 1 and 10 μM significantly unregulated Cu/Zn SOD, CAT, HO-1 and Nrf2 mRNA expression compared to I/R group (Figure 6).
Figure 6.
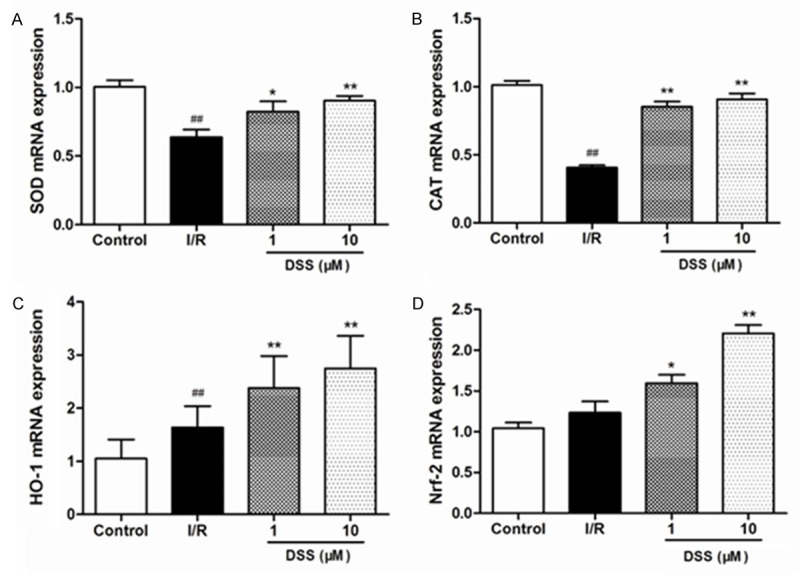
Evaluation of anti-oxidant enzymes by RT-PCR after 30 min reperfusion in isolated hearts pretreated with DSS at 1 and 10 μM. The data were normalized to GAPDH. A. The expression of Cu/Zn SOD mRNA; B. The expression of CAT mRNA; C. The expression of HO-1 mRNA; D. The expression of Nrf2 mRNA. Data expressed as mean ± SD, n = 6. #P < 0.05, ##P < 0.01 versus Control group. **P < 0.01 versus I/R group.
DSS activated the Nrf2 signaling pathway which was mediated by Akt and ERK1/2
Ischemia/reperfusion injury can also activate the transcription factors, crucial regulators against oxidative stress, which regulates ROS detoxifying and antioxidant genes responsible in controlling the mitochondrial redox homeostasis. To explore the molecular mechanism in which DSS can ameliorate the protection against ischemia reperfusion injury, the potential involvement of the well-known Akt, ERK1/2 and Nrf2 pathways was confirmed by western blot analysis in Figure 7. Myocardial samples taken from left ventricular on pretreatment with DSS revealed a significant increase of Akt and ERK1/2 phosphorylated and Nrf2 levels compared to I/R group. This data confirmed the findings that DSS activated Nrf2 signaling pathway via the Akt and ERK1/2 to protect hearts against ischemia reperfusion injury.
Figure 7.
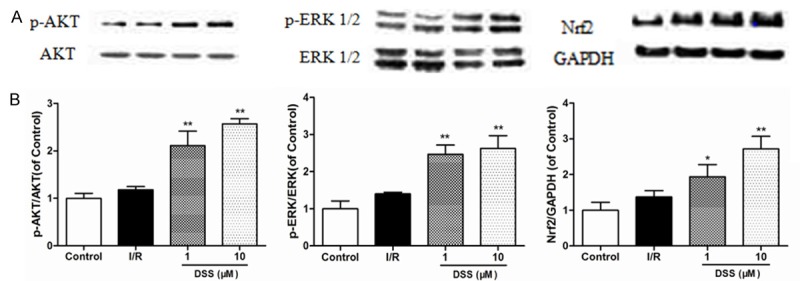
Western blotting results of Akt, p-Akt, ERK1/2, p-ERK1/2 and Nrf2 in different groups. A. Representatives of Western blotting. B. Expressions of phosphorylated Akt, ERK1/2 and Nrf2. Data expressed as mean ± SD, n = 3. #P < 0.05, ##P < 0.01 versus Control group. *P < 0.05, **P < 0.01 versus I/R group.
Discussion
The recovery of cardiac function, myocardial enzyme release and infarct size measurement have been considered endpoints for ischemia reperfusion injury evaluation. Pretreatment with DSS however resulted in decreased I/R injury, intracellular calcium influx and excess ROS generation, this led to an improved cardiac function. In the present study, this was reflected by the reducing necrotic damage (LDH and CK release) and decreasing infarct size. Without such protection, there will be additional necroses cells, leading to plasma membrane disruption, ions homeostasis loss and myocardiac enzymes release. Cardiodynamics, including HR, LVDP and ± dp/dt max, alongside with I/R injury measurement are considered improvement parameters of left ventricular function in isolated rat hearts [37,38]. From this study, HR, LVDP and the recovery rate of ± dp/dt max at the end of reperfusion period were increased significantly by DSS pretreatment. These data suggested that DSS could ameliorate cardiac function after I/R injury.
In another work, Reactive Oxygen Species overproduction was found to be deleterious to the heart and could lead to myocardial I/R injury. At this process, low levels of superoxide anion, hydrogen peroxide, hydroxyl radical and NADPH oxidizes damage the electron transport chain in the myocardium. During early reperfusion, the presence of oxygen enhances, a large burst of ROS which plays a pivotal role in the reperfusion and this has contributed to deleterious oxidative stress [6,39].
Several studies have shown that the myocardium possesses ROS scavengers or endogenous antioxidant defenses such as SOD, GSH-PX, and CAT that could detoxify ROS and attenuate ischemia-reperfusion injury [40,41]. On DSS pretreatment, especially at 10 μM, MDA levels significantly decreased compared to I/R group, while the activity of SOD and GSK-PX increased. This implies that DSS have cardio protective effect on isolated heart against oxidative stress during I/R injury.
Nrf2 signaling pathway is a very important pathway studied so far in preventing cell damage caused by oxidative stress. Under oxidative stress conditions, Nrf2 accumulates and translocates into the nucleus where it markedly up-regulates expression of antioxidant response element (ARE) sequence of phase II detoxifying and antioxidant enzyme genes, including heme oxygenase (HO-1), quinone oxidoreductase 1 (NQO1), glutathione peroxidase (GPX), SOD and CAT. They therefore play a major role antioxidant defense and detoxification of ROS produced during I/R [42-44]. In this study, DSS at 1 and 10 μM significantly enhanced Cu/Zn SOD, CAT, HO-1 and Nrf2 mRNA expression compared to I/R group. This data suggested that DSS can protect against I/R injury via the upregulation of antioxidant defense genes.
Other studies had substantially demonstrated that activation of the Reperfusion Injury Salvage Kinase (RISK) pathway which is a group of survival protein kinases including PI3K/Akt and ERK1/2 known to possess cardioprotection activity, when specifically activated during myocardial reperfusion. Akt, one of the Protein Kinases B (PKB) is best described as survival kinases that is activated by receptor ligands, and its phosphorylation preserves mitochondrial integrity thereby protecting the cardiac cells against necrosis and apoptosis [45]. Phosphatidylinositol 3-kinase (PI3K)/Akt cascade during reperfusion have been proposed to play a key role in IP-mediated cardioprotection [46].
Evidence shows that the activation of extracellular signaling-regulated kinase1/2 (ERK1/2) signaling pathway during ischemia/reperfusion. ERK1/2 activation suppresses apoptosis and promotes cell survival and then protect impaired cardiac function and cardiac injury [47,48].
The PI3K/Akt and ERK1/2 pathway both play critical roles in promoting cell survival in the heart, which are known to modify Nrf2 activity and enhance its translocation into nucleus in response to oxidative stress [49,50]. The previous study shown that cardio protective effects of Danshensu and paeonol combination on isoproterenol-induced myocardial infarction in rats by the enhancing their antioxidant defense system through activating of Nrf2 signaling [51]. Another study suggested that Danshensu could provide significant cardioprotection against MI/R injury, and the potential mechanisms might to suppression of cardiomyocytes apoptosis through activating the PI3K/Akt and ERK1/2 signaling pathways [22]. From our investigation, myocardial samples taken from left ventricular pretreated with DSS revealed a significant increase in Akt and ERK1/2 phosphorylation and Nrf2 levels. These findings suggested that the underlying mechanisms of the cardioprotective effects of DSS involved activation of Akt/ERK1/2/Nrf2 signaling during ischemia reperfusion.
This study demonstrated a cardioprotective effect of Danshensu on isolated heart against oxidative stress during ischemia reperfusion injury, and the underlying mechanisms might be associated with the enhancement of antioxidant defense system by activating Akt/ERK1/2/Nrf2 signaling pathways.
Acknowledgements
We thank the financial supports from the National Key Basic Research Program of China (2012CB518400), the National Natural Science Foundation of China (81001659, 81273891), National Science Fund for Distinguished Young Scholars (81125024), and Technology major projects (2012ZX09103201-046).
Disclosure of conflict of interest
None.
References
- 1.Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68–78. [PubMed] [Google Scholar]
- 2.Magro M, Garg S, Serruys PW. Revascularization treatment of stable coronary artery disease. Expert Opin Pharmacother. 2011;12:195–212. doi: 10.1517/14656566.2010.517522. [DOI] [PubMed] [Google Scholar]
- 3.Minamino T. Cardioprotection from ischemia/ reperfusion injury: basic and translational research. Circ J. 2012;76:1074–82. doi: 10.1253/circj.cj-12-0132. [DOI] [PubMed] [Google Scholar]
- 4.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–58. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 5.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–58. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 6.Mozaffari MS, Liu JY, Abebe W, Baban B. Mechanisms of load dependency of myocardial ischemia reperfusion injury. Am J Cardiovasc Dis. 2013;3:180–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–8. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stark G, Huber U, Hofer E, Tritthart HA. Continuous ECG measurements of intracardiac activity from the surface of Langendorffperfused guinea pig hearts. Basic Res Cardiol. 1987;82:437–44. doi: 10.1007/BF01907091. [DOI] [PubMed] [Google Scholar]
- 9.Tanguay M, Blaise G, Dumont L, Beique G, Hollmann C. Beneficial effects of volatile anesthetics on decrease in coronary flow and myocardial contractility induced by oxygen-derived free radicals in isolated rabbit hearts. J Cardiovasc Pharmacol. 1991;18:863–70. doi: 10.1097/00005344-199112000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Pasdois P, Parker JE, Griffiths EJ, Halestrap AP. Hexokinase II and reperfusion injury: TAT-HK2 peptide impairs vascular function in Langendorff-perfused rat hearts. Circ Res. 2013;112:e3–7. doi: 10.1161/CIRCRESAHA.112.274233. [DOI] [PubMed] [Google Scholar]
- 11.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 2011;50:940–50. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Chan SW, Tseng HL, Deng Y, Hoi PM, Choi PS, Or PM, Yang JM, Lam FF, Lee SM, Leung GP, Kong SK, Ho HP, Kwan YW, Yeung JH. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine. 2012;19:1263–9. doi: 10.1016/j.phymed.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Zhao GR, Zhang HM, Ye TX, Xiang ZJ, Yuan YJ, Guo ZX, Zhao LB. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol. 2008;46:73–81. doi: 10.1016/j.fct.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Zhao QT, Guo QM, Wang P, Wang Q. Salvianic acid A inhibits lipopolysaccharide-induced apoptosis through regulating glutathione peroxidase activity and malondialdehyde level in vascular endothelial cells. Chin J Nat Med. 2012;10:53–7. doi: 10.1016/S1875-5364(12)60012-0. [DOI] [PubMed] [Google Scholar]
- 15.Lu H, Tian A, Wu J, Yang C, Xing R, Jia P, Yang L, Zhang Y, Zheng X, Li Z. Danshensu inhibits beta-adrenergic receptors-mediated cardiac fibrosis by ROS/p38 MAPK axis. Biol Pharm Bull. 2014;37:961–7. doi: 10.1248/bpb.b13-00921. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Xu Y, Wang J, Zhang K, Yi B, Liu Y, Cai X. [Effect of Danshensu on fibronectin and collagen-1 secretion induced by high glucose in human peritoneal mesothelial cells] . Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:44–50. doi: 10.3969/j.issn.1672-7347.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Ha T, Wei D. [A study on the mechanism of the biological roles of danshensu on fibroblast] . Zhonghua Shao Shang Za Zhi. 2001;17:36–8. [PubMed] [Google Scholar]
- 18.Yang GD, Zhang H, Lin R, Wang WR, Shi XL, Liu Y, Ji QL. Down-regulation of CD40 gene expression and inhibition of apoptosis with Danshensu in endothelial cells. Basic Clin Pharmacol Toxicol. 2009;104:87–92. doi: 10.1111/j.1742-7843.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- 19.Guan Y, Yin Y, Zhu YR, Guo C, Wei G, Duan JL, Wang YH, Zhou D, Quan W, Weng Y, Xi MM, Wen AD. Dissection of mechanisms of a chinese medicinal formula: danhong injection therapy for myocardial ischemia/reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2013;2013:972370. doi: 10.1155/2013/972370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao BL, Jiang W, Zhao Y, Hou JW, Xin WJ. Scavenging effects of salvia miltiorrhiza on free radicals and its protection for myocardial mitochondrial membranes from ischemia-reperfusion injury. Biochem Mol Biol Int. 1996;38:1171–82. [PubMed] [Google Scholar]
- 21.Xiao-Yong L, CCL , ML , et al. Effects of Danshensu on the Incidence of Ischemiareperfusion Induced Arrhythmia in Hypertrophy Rat Heart. Chinese Journal of Natural Medicines. 2008;6:461–5. [Google Scholar]
- 22.Yin Y, Guan Y, Duan J, Wei G, Zhu Y, Quan W, Guo C, Zhou D, Wang Y, Xi M, Wen A. Cardioprotective effect of Danshensu against myocardial ischemia/reperfusion injury and inhibits apoptosis of H9c2 cardiomyocytes via Akt and ERK1/2 phosphorylation. Eur J Pharmacol. 2013;699:219–26. doi: 10.1016/j.ejphar.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Guo ZQ, Wang SR, Zhu LQ. [Effect of danshensu and ligustrazine on related genes of myocardial hypertrophy induced by angiotensin II] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:342–4. [PubMed] [Google Scholar]
- 24.Tang Y, Wang M, Le X, Meng J, Huang L, Yu P, Chen J, Wu P. Antioxidant and cardioprotective effects of Danshensu (3-(3, 4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in rats. Phytomedicine. 2011;18:1024–30. doi: 10.1016/j.phymed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Yang RX, Huang SY, Yan FF, Lu XT, Xing YF, Liu Y, Zhao YX. Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol Sin. 2010;31:1395–400. doi: 10.1038/aps.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Bao Y, Yang Y, Wu Y, Chen X, Si S, Hong B. Discovery of antagonists for human scavenger receptor CD36 via an ELISA-like highthroughput screening assay. J Biomol Screen. 2010;15:239–50. doi: 10.1177/1087057109359686. [DOI] [PubMed] [Google Scholar]
- 27.de Vries DK, Kortekaas KA, Tsikas D, Wijermars LG, van Noorden CJ, Suchy MT, Cobbaert CM, Klautz RJ, Schaapherder AF, Lindeman JH. Oxidative damage in clinical ischemia/reperfusion injury: a reappraisal. Antioxid Redox Signal. 2013;19:535–45. doi: 10.1089/ars.2012.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilgore KS, Lucchesi BR. Reperfusion injury after myocardial infarction: the role of free radicals and the inflammatory response. Clin Biochem. 1993;26:359–70. doi: 10.1016/0009-9120(93)90112-j. [DOI] [PubMed] [Google Scholar]
- 29.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation. 1989;80:1115–27. doi: 10.1161/01.cir.80.5.1115. [DOI] [PubMed] [Google Scholar]
- 30.Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev. 2013;41:162–8. doi: 10.1097/JES.0b013e3182948a1e. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, Trush MA, Misra HP, Li Y. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8:71–85. doi: 10.1007/s12012-008-9016-0. [DOI] [PubMed] [Google Scholar]
- 32.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–74. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peake BF, Nicholson CK, Lambert JP, Hood RL, Amin H, Amin S, Calvert JW. Hydrogen sulfide preconditions the db/db diabetic mouse heart against ischemia-reperfusion injury by activating Nrf2 signaling in an Erk-dependent manner. Am J Physiol Heart Circ Physiol. 2013;304:H1215–24. doi: 10.1152/ajpheart.00796.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li YH, Yu B, Duan ZZ, Akinyi OM, Yu JH, Zhou K, Zhang Y, Gao XM. The coronary dilation effect of shen fu injection was mediated through NO. PLoS One. 2014;9:e92415. doi: 10.1371/journal.pone.0092415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrera R, Bopassa JC, Angoulvant D, Ovize M. Post-conditioning protects from cardioplegia and cold ischemia via inhibition of mitochondrial permeability transition pore. J Heart Lung Transplant. 2007;26:604–9. doi: 10.1016/j.healun.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Ito WD, Schaarschmidt S, Klask R, Hansen S, Schafer HJ, Mathey D, Bhakdi S. Infarct size measurement by triphenyltetrazolium chloride staining versus in vivo injection of propidium iodide. J Mol Cell Cardiol. 1997;29:2169–75. doi: 10.1006/jmcc.1997.0456. [DOI] [PubMed] [Google Scholar]
- 37.Maslov LN, Lasukova OV, Krylatov AV, Hanus LO, Pertwee R, Ivanchuk II, Crowford D. Role of cannabinoid receptors in the regulation of cardiac contractility during ischemia/reperfusion. Bull Exp Biol Med. 2006;142:557–61. doi: 10.1007/s10517-006-0417-4. [DOI] [PubMed] [Google Scholar]
- 38.Kolettis TM, Kontaras K, Spartinos I, Maniotis C, Varnavas V, Koutouzis M, Mourouzis I, Papalois A, Pantos C, Kyriakides ZS. Dosedependent effects of sildenafil on post-ischaemic left ventricular function in the rat isolated heart. J Pharm Pharmacol. 2010;62:346–51. doi: 10.1211/jpp.62.03.0009. [DOI] [PubMed] [Google Scholar]
- 39.Mineo TC, Tacconi F. From “awake” to “monitored anesthesia care” thoracic surgery: A 15 year evolution. Thoracic Cancer. 2014;5:1–13. doi: 10.1111/1759-7714.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steare SE, Yellon DM. The potential for endogenous myocardial antioxidants to protect the myocardium against ischaemia-reperfusion injury: refreshing the parts exogenous antioxidants cannot reach? J Mol Cell Cardiol. 1995;27:65–74. doi: 10.1016/s0022-2828(08)80008-9. [DOI] [PubMed] [Google Scholar]
- 41.Leichtweis S, Ji LL. Glutathione deficiency intensifies ischaemia-reperfusion induced cardiac dysfunction and oxidative stress. Acta Physiol Scand. 2001;172:1–10. doi: 10.1046/j.1365-201X.2001.00820.x. [DOI] [PubMed] [Google Scholar]
- 42.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O’Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–6. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 43.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 44.Chen EP, Bittner HB, Davis RD, Van Trigt P, Folz RJ. Physiologic effects of extracellular superoxide dismutase transgene overexpression on myocardial function after ischemia and reperfusion injury. J Thorac Cardiovasc Surg. 1998;115:450–8. doi: 10.1016/s0022-5223(98)70289-2. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Chanoit G, McIntosh R, Zvara DA, Xu Z. Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2009;297:H569–75. doi: 10.1152/ajpheart.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–53. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Fan H, Yang L, Fu F, Xu H, Meng Q, Zhu H, Teng L, Yang M, Zhang L, Zhang Z, Liu K. Cardioprotective effects of salvianolic Acid a on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2012;2012:508938. doi: 10.1155/2012/508938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovacs K, Hanto K, Bognar Z, Tapodi A, Bognar E, Kiss GN, Szabo A, Rappai G, Kiss T, Sumegi B, Gallyas F Jr. Prevalent role of Akt and ERK activation in cardioprotective effect of Ca(2+) channel- and beta-adrenergic receptor blockers. Mol Cell Biochem. 2009;321:155–64. doi: 10.1007/s11010-008-9929-8. [DOI] [PubMed] [Google Scholar]
- 49.Cui G, Shan L, Hung M, Lei S, Choi I, Zhang Z, Yu P, Hoi P, Wang Y, Lee SM. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol. 2013;168:1349–59. doi: 10.1016/j.ijcard.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Lee YJ, Jeong HY, Kim YB, Won SY, Shim JH, Cho MK, Nam HS, Lee SH. Reactive oxygen species and PI3K/Akt signaling play key roles in the induction of Nrf2-driven heme oxygenase-1 expression in sulforaphane-treated human mesothelioma MSTO-211H cells. Food Chem Toxicol. 2012;50:116–23. doi: 10.1016/j.fct.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Xie YH, Yang Q, Wang SW, Zhang BL, Wang JB, Cao W, Bi LL, Sun JY, Miao S, Hu J, Zhou XX, Qiu PC. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS One. 2012;7:e48872. doi: 10.1371/journal.pone.0048872. [DOI] [PMC free article] [PubMed] [Google Scholar]


