Abstract
Zhenrenyangzang Decoction (ZD) has been used as a classic formula in China for the treatment of gastrointestinal dysfunction such as chronic gastritis. However, there is less study on its application in ulcerative colitis (UC) and the effects are not yet clearly defined. To explore the effectiveness of ZD in trinitrobenzene sulfonic acid (TNBS)-induced UC rats, ZD was administered orally for 8 days at a dosage of 2, 4 or 8 g/kg/day. Following drug administration, the disease activity index (DAI) and tissue damage scores were recorded. In addition, mRNA and protein expression of nuclear factor kappa B (NF-κB), p38 mitogen activated protein kinase (p38MAPK) and Toll-like receptor 2 (TLR2) in colon tissues were examined by real time PCR and western blotting assay. As compared with the UC model group, ZD promoted the recovery of colitis and inhibited the colonic inflammation damage in UC rats by reducing the mRNA or protein expression of NF-κB and p38MAPK, as well as activating the production of TLR2 in colon tissues. And ZD significantly reduced the DAI and tissue damage scores. The therapeutic effect of ZD was found to be comparable to that of SASP. Our results suggested that ZD could improve colonic mucosa impairment and possesses favorable therapeutic action in TNBS-induced colitis, which provides direct pharmacological evidence for its clinical application.
Keywords: Zhenrenyangzang decoction, ulcerative colitis, therapy
Introduction
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease of the colon characterized clinically by diarrhea, abdominal pain and mucuspus stool. It is reported that factors such as immune disturbance, heredity, inflammatory cytokines and environment, etc. play a crucial role in the development of UC [1]. The incidence and prevalence of UC are now increasing worldwide. In recent years, the incidence rates appear to be increasing in developing countries in Europe and Asia with westernization of lifestyle and industrialization, including China, South Korea and India [2]. Available therapies for UC include conventional anti-inflammatory agents (such as 5-aminosalicylates and corticosteroids), immune modulators and biological therapy. Biological therapy also aims at antagonizing pro-inflammatory molecules. However, the current anti-inflammatory therapy (e.g., treatment with anti-tumor necrosis factor (TNF)-α antibody) does not cure the disease and results in long-term remission only in fewer than 34% of patients [3]. Hence, anti-inflammatory therapy is not completely effective in eliminating the disease, indicating that other pathogenic factors may play important roles in UC [4]. Furthermore, UC management requires long-term treatment that often leads to drug refractoriness or intolerance [5]. Patients who are unresponsive to the current therapy still suffer from this common disease. Therefore, it is necessary to develop novel therapeutic approaches.
Complementary and alternative medicine (CAM) is becoming increasingly popular and is used to treat patients with UC in China. Physicians and patients consider CAM as an effective adjunct treatment [6]. Zhenrenyangzang decoction (ZD), containing ten commonly used plants (ginseng, angelica, muscade, cinnamon, glycyrrhiza, etc.), is a canonical Chinese medicine prescription. This traditional Chinese medicine formulation is a promising agent for the treatment of many chronic gastrointestinal diseases, including chronic gastritis, peptic ulcer disease and certain chronic intestinal diseases [7]. However, the use of ZD for the treatment of UC has little to be reported, and the pharmacological mechanism is not yet clearly defined.
Therefore, the present study was designed to explore the effects of oral administration of ZD on 2,4,6-tri-nitrobenzenesulfonic acid (TNBS)-induced UC rats by examining the level of nuclear factor kappa B (NF-κB), p38 mitogen activated protein kinase (p38MAPK) and Toll-like receptor 2 (TLR2) in colon.
Materials and methods
Preparation of modified Zhenrenyangzang decoction (ZD)
Herbal materials were obtained from Qiqihar Hospital of Traditional Chinese Medicine. ZD were prepared by grinding and mixing of the 9 component dried raw herbs (Ginsneg, Angelica Sinensis, Rhizoma Atractylodis Macrocephalae, Nutmeg, Cinnamon, Radix Glycyrrhizae, Radices Paeoniae Alba, Radices Saussureae and Chebule) in proportions as shown in Table 1. Herbs were decocted with boiling water at 100 g/L for 60 min twice. Decoctions were then filtered and pooled. For the purpose of oral feeding to mice in a smaller volume, the pooled decoction was further concentrated and freeze-dried.
Table 1.
Composition of herbs in the formulation ZD on a dry weight basis
| Chinese herbs | Alias | Composition (%) |
|---|---|---|
| Ginsneg | RenShen | 11.36 |
| Angelica Sinensis | Dang Gui | 11.36 |
| Rhizoma Atractylodis Macrocephalae | BaiShu | 13.63 |
| Nutmeg | Rou Doukui | 13.63 |
| Cinnamon | Rou Gui | 3.41 |
| Radix Glycyrrhizae | ZhiGancao | 6.82 |
| Radices Paeoniae Alba | Bai Shao | 17.04 |
| Radices Saussureae | Mu Xiang | 11.36 |
| Chebule | He Zi | 11.36 |
Animals and ethical approval
Eight weeks old male Sprague Dawley (SD) rats weighting 200-240 g were received from Experimental Animal Care Center, Harbin Medical University, China (License No. SCXK 2013-001). Animals were housed under controlled environmental conditions (25°C and a 12 h light/dark cycle). All experimental procedures and protocols in this study including euthanasia were conducted in accordance with the Ethical Guidelines of the Experimental Animal Care Center, Qiqihar Medical University, China.
Induction of UC model and drug treatment
Experimental colitis was induced according to a modification of the procedure [8]. Prior to induction, all rats were fasted overnight but given free access to water. After anaesthetized with 30 mg/kg pentobarbital sodium, the rats were received a single rectal injection of TNBS/ethanol mixture (70 mg/kg TNBS diluted in 0.25 mL of 50% ethanol) slowly through a catheter with 2 mm diameter at the depth of 8 cm from rectal sphincter, and then the rats were left for 15 min in a supine Trendelenburg position with the anus clipped. Seventy TNBS-induced rats were randomly divided into five groups: low, middle and high dosages of ZD groups (2, 4 and 8 g/kg, respectively), salazosulfapyridine (SASP) group (0.5 g/kg), and model group (an equal volume of saline). Additionally, fifteen rats used as normal control group were rectally injected with saline instead of TNBS. The animals were administered by intragastric gavage once a day, and continuously for 8 days.
Histopathological investigations
Colon sections were fixed 10% neutral buffered formalin then put for 24 h in decal. Samples were then cut into several sections and embedded into paraffin wax blocks. Tissues were stained with haematoxylin and eosin and were mounted and observed microscopically for histopathological changes by a pathologist in blinded fashion.
Macroscopic and histological evaluation of colonic damage
A 10-cm segment of the distal colon was removed for the morphological study. Colonic mucosa damage was assessed according to previously described macroscopic scoring system [9] as follows: 0= normal mucosa; 1= localized hyperemia but no erosions, ulcers, or scars; 2= linear ulcer or scar with inflammation at one site >2 mm but <5 mm; 3= two or more sites of ulceration and/or inflammation, each up to 5 mm; 4= two or more major sites of inflammation and ulcerations >5 mm each or one major site of inflammation extending >1 cm along the length of the mucosa. The tissue fragments (2×10 mm2) were excised from the central part of the lesion of each colon, fixed in 4% polyformaldehyde prior to wax embedding, sectioning, and staining with hematoxylin and eosin (HE). Histological scores were performed by a pathologist in a blind method using the criteria [10]. Each section was graded with a range from 0 to 4 as to depth of the lesion, extent of ulceration and with a range from 0 to 3 as to degree of inflammation. These changes were indicated according to the following scale: depth of the lesion, 0= none, 1= mucosa, 2= submucosa, 3= muscularispropria, 4= serosa; degree of inflammation, 0= none, 1= slight, 2= moderate, 3= severe; extent of ulceration, 0= none, 1= mild surface (0-25%), 2= moderate surface (25-50%), 3= severe surface (50-75%), 4= extensive-full thickness (more 75%).
Disease activity index (DAI) analysis
The DAI was determined at the end of the treatment period using the DAI scoring system described in the previous report [11]. The scores for stool consistency and occult blood for each rat were added and then given a DAI score for each rat. Each score was determined as follows: stool consistency (0 and 1: normal, 2 and 3: loose stool, 4: diarrhea) and stool blood (0: negative, 1: ±, 2: +, 3: ++, 4: gross).
Real time PCR analysis mRNA expression
mRNA was extracted from colonic tissue samples using Trizol according to the manufacturer’s protocols (Invitrogen) and RT-PCR was performed according to the instructions of the Takara RNA PCR kit 3.0 (AMV). An equal amount of cDNA from each sample was amplified using primers specific to each gene (Table 2). Real time quantitative PCR was performed in a 25 μl final volume containing the 2 X SYBR Green I master mix (Qiagen, Hilden, Germany). DNA amplification was done using a thermocycler under the following conditions: 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 90 s. The relative quantification of expression of the gene was normalized to the internal control gene actin and determined using the 2-ΔΔCt method as described [12]. The fold changes of the treated over the control group were calculated.
Table 2.
Primers for real time PCR
| Gene | Primer sequence | PCR product (bp) |
|---|---|---|
| NF-kB | 5’ AGCACTGTGAGGACGGCATA 3’ | 84 |
| 5’ CGTGAAGTATTCCCAGGTTTG 3’ | ||
| P38MAPK | 5’ TAGACGAATGGAAGAGCCTGAC 3’ | 168 |
| 5’ GGCACTTGAATGGTATTTGGAG 3’ | ||
| TLR2 | 5’ AAGTAGAAACGGTAACAATACGGAG 3’ | 144 |
| 5’ AGAAAGAGCAGGGAACCAGAA 3’ | ||
| Actin | 5’CGTAAAGACCTCTATGCCAACA 3’ | 163 |
| 5’ AGCCACCAATCCACACAGAG 3’ |
Western blotting assay
Proteins (50 μg) that were extracted from colonic mucosal scrapings were subjected to SDS-polyacrylamide gel electrophoresis after dissociation by boiling (5 min) in 2 X loading buffer (0.125 M Tris/HCl, pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.05% bromphenol blue). After electrophoresis proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories). The membranes were incubated with 1:200 rabbit polyclonal IκB-α antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1:500 mouse monoclonal phosphorylated p38MAPK antibody (Santa Cruz Biotechnology, Inc.), 1:200 mouse monoclonal TLR2 antibody (Santa Cruz Biotechnology, Inc.). Loading control was performed by using a mouse monoclonal antibody to GAPDH (1:2000; Santa Cruz Biotechnology, Inc.). The density of Western blotting bands was measured by using the Meta Morph 7.5 Video image Analysis System (Molecular Devices) and presented as relative density against density of GAPDH bands.
Statistical analysis
Data were presented as mean ± standard deviation. Differences among groups were analyzed using one-way ANOVA with SPSS 11.0 software. P<0.05 was considered statistically significant.
Results
ZD prevents TNBS-induced histopathological change in UC rats
In the normal group, the colonic mucosa was intact. The submucosal muscle and mucosal epithelium was continuous and integrated. In the model group, the colon lumen dilated and colonic wall thickened, and several irregular ulcers were accompanied by obviously peripheral mucosal hyperemia and edema. Tissue adherence and scar were also found in model rats. The tissue of model rats showed neutrophils, eosinophils and lymphocytes infiltration. In mucosa, part of glands missed, goblet cells reduced, irregular ulcer and inflammatory granuloma formed. In contrast, the ulcer area significantly reduced and edema disappeared in the SASP and ZD treated groups. And the high dose of ZD group more greatly relieved the lesions than the low dose group (Figure 1).
Figure 1.
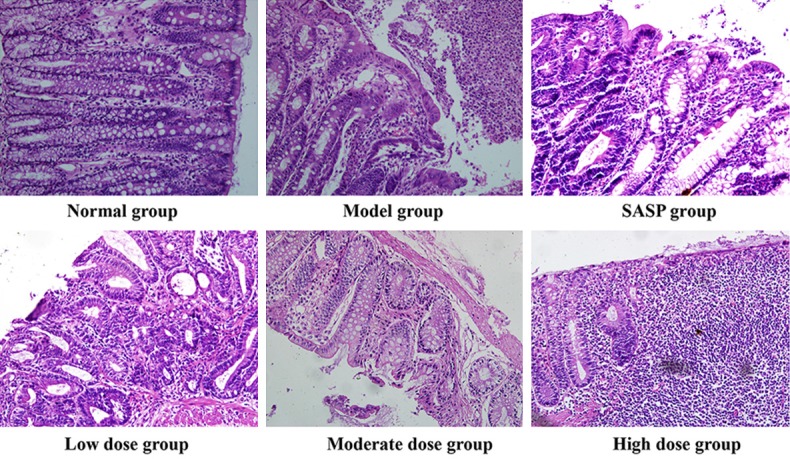
Histological change of colon tissues in trinitrobenzenesulfonic acid (TNBS)-induced UC rats after orally administration of low dose, moderate dose or high dose of ZD (2, 4 and 8 g/kg), salicylazosulfapyridine (SASP, 0.5 g/kg) or saline daily for 8 days (hematoxylin and eosin stain, ×200).
ZD improves clinical symptoms in UC rats
After administration of TNBS, animals developed colitis associated with soft stool and diarrhea (Table 3). All TNBS-treated rats (Model group) had profound weight loss compared with the weight obtained in normal group (P<0.01). Treatment with SASP or different dose of ZD gradually recovered the lost body weight beginning on day 3, accompanied by alleviating symptoms (Figure 2; Table 3).
Table 3.
Evaluation of stool consistency after TNBS induction and ZD treatment in UC rats
| Stool Consistency | Day 2 | Day 5 | Day 8 |
|---|---|---|---|
| Normal | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Model | 1.15 ± 0.45* | 2.25 ± 0.25** | 2.85 ± 0.36** |
| SASP | 1.15 ± 0.5** | 2.10 ± 0.35* | 1.15 ± 0.15** |
| Low dose | 1.15 ± 0.42* | 2.20 ± 0.35* | 2.55 ± 0.35* |
| Moderate dose | 1.10 ± 0.35* | 2.05 ± 0.25* | 2.15 ± 0.25* |
| High dose | 1.05 ± 0.25** | 1.90 ± 0.15* | 1.10 ± 0.25** |
Scoring of stool consistency: 0= no diarrhea; 1= mild; 2= severe; 3= severe and bloody diarrhea. The scores of each group were the average of 6 animals.
P<0.05 compared to model group.
P<0.01 compared to model group.
ZD, Zhenrenyangzang decoction; SASP, salazosulfapyridine.
Figure 2.
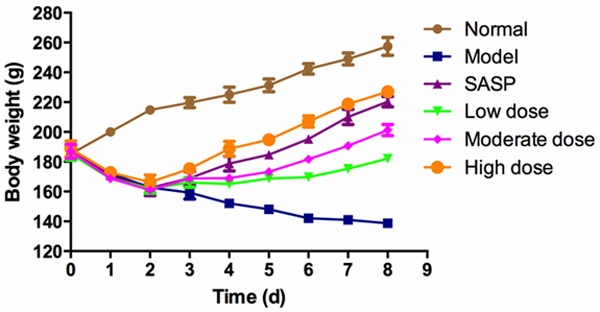
Treatment with ZD ameliorates body weight loss in TNBS-induced UC rats. Colitis was induced by TNBS, then rats were treated daily for 8 days with different doses of ZD (2, 4 and 8 g/kg) and SASP (0.5 g/kg). Disease progression was monitored by observation of body weight change. Data are represented as mean ± SD of 14 animals of each group.
ZD reduces colonic macroscopic damage and DAI scores in UC rats
Control normal animals showed no colonic damage and the colonic damage score was zero. Compared to the normal group, the macroscopic and histological damage scores of colon tissues in the model group significantly increased (P<0.01). Moreover, the scores were significantly lower in the SASP and ZD groups compared with the model group (P<0.05). The scores in the SASP group were lower than that in the ZD groups (P<0.05). And significant changes have been observed in the different doses of ZD groups (P<0.05, Table 4).
Table 4.
Effects of ZD on macroscopic and histological damage scores in UC rats
| Group | Dose (g/kg) | Macroscopic score | Histological score |
|---|---|---|---|
| Normal | Saline (10 ml/kg) | 0 ± 0 | 0 ± 0 |
| Model | Saline (10 ml/kg) | 6.15 ± 0.26** | 9.17 ± 0.34** |
| SASP | 0.5 | 2.12 ± 0.31* | 3.15 ± 0.16* |
| High dose | 8 | 2.64 ± 0.27** | 3.42 ± 0.38* |
| Moderate dose | 4 | 4.58 ± 0.39* | 5.31 ± 0.22* |
| Low dose | 2 | 6.02 ± 0.13* | 8.92 ± 0.23* |
P<0.05 compared to model group.
P<0.01 compared to model group.
ZD, Zhenrenyangzang decoction; SASP, salazosulfapyridine.
Also, we evaluated the severity of colitis based on the DAI scores. The higher the DAI score, the more severe the colitis. We found that the DAI scores of the different dose of ZD or SASP groups were significantly lower than those of the model group, and the high dose of ZD is the lowest. In contrast, the DAI score of the normal group was nil (Figure 3). These results suggest that oral administration of ZD or SASP clearly attenuated the impairment to the colon tissues.
Figure 3.
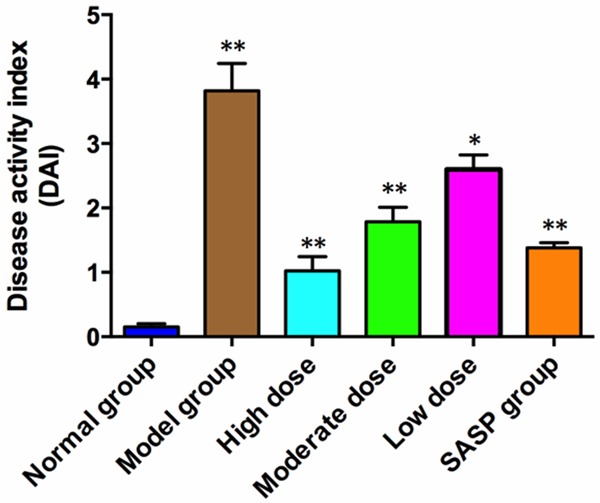
The DAI scores of different groups of animals. The DAI socres were measured after 8 days of treatment. Treatment with SASP or ZD reduced the DAI score, and a significant difference was observed compared with the normal group. *P<0.05; **P<0.01 vs. the normal group.
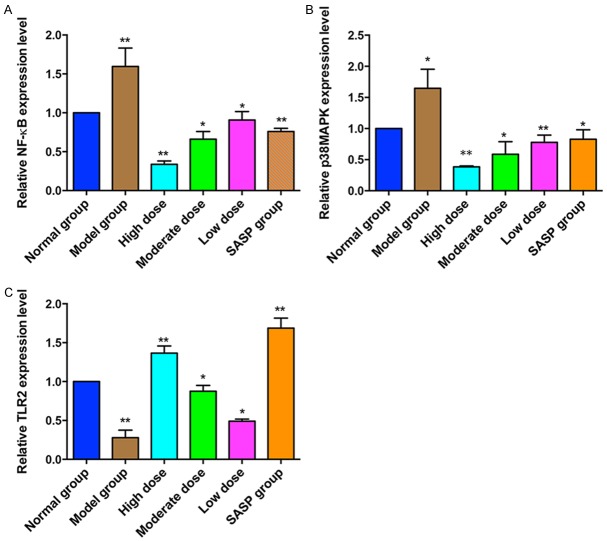
mRNA expression of NF-κB, p38MAPK and TLR2
The expression levels of NF-κB and p38MAPK mRNA were significantly higher in the model group compared with those in the normal group. However, compared to the UC model group, NF-κB and p38MAPK mRNA expression in the ZD or SASP treatment groups were reduced, and the reduction by ZD was dose dependent. In contrast, a significant decrease of TLR2 mRNA expression in TNBS model group compared with the normal control group. But ZD or SASP treatment groups significantly activated the TLR2 mRNA expression comparing to the model group. And the activation of expression by ZD was in a dose dependent manner (Figure 4).
Figure 4.
Real time PCR analysis the mRNA expressions of NF-κB (A), p38MAPK (B) and TLR2 (C) in colonic tissue sections after the treatment of different doses of ZD (2, 4 and 8 g/kg), salicylazosulfapyridine (SASP, 0.5 g/kg) or saline daily for 8 days. Independent triplicate assays were performed for each RNA sample and data was shown as fold changes of expression. The expression value in normal group was set as 1. *P<0.05; **P<0.01 vs. the model group.
IκB-α, p38MAPK and TLR2 protein expression in colon tissues
As depicted in Figure 5, western blotting results showed that TLR2 protein level was obviously higher in ZD-treated rat tissues compared with that in TNBS induced model rats. And the ZD treatment followed a dose-dependent manner. In contrast, administration of ZD attenuated the protein expression of IκB-α subunit and phosphorylated p38MAPK (p-p38MAPK) in comparison with model group. The p-p38MAPK protein level was the lowest in the high dose ZD group. However, there were no obvious differences for the IκB-α protein expression among the low, moderate and high ZD groups (Figure 5).
Figure 5.
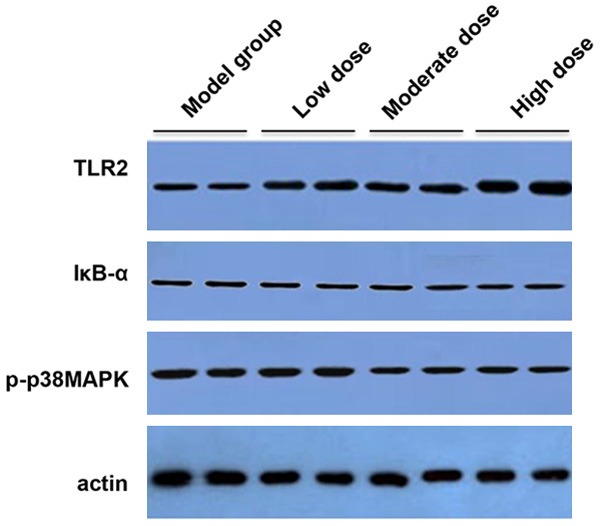
Western blotting analysis the protein expressions of TLR2, IκB-α and phosphorylated p38MAPK (p-p38MAPK) in colonic tissue sections after the treatment of different doses of ZD (2, 4 and 8 g/kg) or saline daily for 8 days. Three independent protein samples from each group were examined.
Discussion
TNBS-induced UC model in present study was widely adopted to mimic acute phase in human UC and assess the effects of drugs [13]. It was characterized by extensive infiltration of inflammatory cells and colonic ulcers. Similar to acute phase, these major histological features also appeared in chronic phase of UC. In this study, our results indicated that oral treatment with ZD significantly promoted the recovery of colitis and inhibited the inflammatory response, which were verified by macroscopic and histological examination, the decreased expression of NF-κB and p38MAPK, as well as the enhanced level of TLR2 in ulcerative colon tissues.
Several agents used in the management of UC, such as corticosteroids, sulfasalazine, and 5-aminosalicylic acid, have been documented to regulate the NF-κB function [14]. NF-κB controls many important biological decisions, from formation of dorsal-ventral polarity in insects to activation of inflammatory and innate immune responses. The key event in NF-κB activation is IκB phosphorylation, the next logical step is to search for a stimulus-responsive IκB kinase (IκK) that could catalyze this event. The activated IκK complexes then phosphorylate IκB subunits in NF-κB: IκB complexes, triggering their ubiquitin-dependent degradation, and the activation of NF-κB [15]. In our study, we measured the mRNA and protein level of NF-κB or IκB-α. Rats in the TNBS model group exhibited higher mRNA levels of NF-κB than those in the normal control group. However, the NF-κB mRNA levels were reduced by ZD treated in a dose-dependent manner, compared with the TNBS model group, the NF-κB mRNA levels in the ZD high dose, moderate dose and SASP groups were obviously downregulated. Moreover, we did not observe obvious change of the IκB-α protein expression among these groups.
Our results also demonstrated that the mRNA and protein levels of (phosphorylated) p38MAPK were significantly decreased in ZD treatment groups comparing with TNBS model group. MAPK family of proteins is the principal regulator of gene expression, and critically controls transcription of a number of cytokine genes. P38MAPK is particularly involved in the inflammatory process, inflammatory stimuli being strong activators of this kinase, and its activation is required for inflammatory gene transcription in vitro [16]. P38MAPK has been reported to contribute to the hypercontractility and increase the Ca2+ sensitization in murine experimental colitis [17]. A specific p38MAPK inhibitor, SB203580, was found to have a dichotomal effect in TNBS induced mice. Treatment with SB203580 does not ameliorate TNBS colitis although it does prevent IFN-γ and IL-12p70 production [18]. This indicates that p38 MAPK may have a broader role in the mucosal immune response and is not only responsible for the production of proinflammatory cytokines but may also be involved in counter regulatory responses.
We also examined the mRNA and protein expression levels of TLR2 in ZD groups and TNBS model group. The Toll-like receptors (TLRs) are key regulators of the innate immune system in the gut through the induction of pro-inflammatory and immune modulatory responses in many cell types including immune and epithelial cells [19]. TLR1 to 9 have been reported detectable in human intestine at least in the mRNA levels both in healthy and disease conditions [20]. In particular TLR2 and TLR4 mRNA and protein have been reported to be upregulated in UC and in other intestinal inflammatory conditions [21-24]. Consistent with the previous findings, by qPCR and western blotting assay we showed that the level of TLR2 mRNA and protein in colonic tissues from ZD groups were significantly higher than that from UC model group. Increased TLR2 expression may enhance the recognition and presentation of antigens by inflammatory cells, rendering the normal bacterial flora to be identified, thereby breaking immune tolerance and leading to intestinal injury.
In conclusion, our results showed that treatment with ZD enema attenuates the clinical symptoms of TNBS-induced colitis, and ZD has a significant curative effect through activating TLR2 as well as inhibiting NF-κB and p38MAPKlevels in UC rats. These results provide supporting evidence for the clinical application of ZD in UC. Protective factors will also be examined in the future in order to elucidate the therapeutic mechanism of ZD enema.
Acknowledgements
This work was supported by the Education Department of Heilongjiang Province Science and Technology Research Projects under Grant No. 12531798.
Disclosure of conflict of interest
None.
References
- 1.Kho YH, Pool MO, Jansman FG, Harting JW. Pharmacotherapeutic options in inflammatory bowel disease: an update. Pharm World Sci. 2001;23:17–21. doi: 10.1023/a:1011268302386. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L. Anti-TNF therapy in inflammatory bowel diseases: a huge review. Minerva Gastroenterol Dietol. 2010;56:233–243. [PubMed] [Google Scholar]
- 4.McLean LP, Cross RK. Adverse events in IBD: to stop or continue immune suppressant and biologic treatment. Expert Rev Gastroenterol Hepatol. 2014;8:223–240. doi: 10.1586/17474124.2014.881715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711–1747. doi: 10.1089/ars.2012.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langhorst J, Wulfert H, Lauche R, Klose P, Cramer H, Dobos GJ, Korzenik J. Systematic Review of Complementary and Alternative Medicine Treatments in Inflammatory Bowel Diseases. J Crohns Colitis. 2015;9:86–106. doi: 10.1093/ecco-jcc/jju007. [DOI] [PubMed] [Google Scholar]
- 7.Chen ZS, Nie ZW, Sun QL. Clinical study in treating intractable ulcerative colitis with traditional Chinese medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1994;14:400–402. [PubMed] [Google Scholar]
- 8.Buell MG, Berin MC. Neutrophilindependence of the initiation of colonic injury. Comparison of results from three models of experimental colitis in the rat. Dig Dis Sci. 1994;39:2575–2588. doi: 10.1007/BF02087693. [DOI] [PubMed] [Google Scholar]
- 9.Hara DB, Fernandes ES, Campos MM, Calixto JB. Pharmacological and biochemical characterization of bradykinin B2 receptors in the mouse colon: influence of the TNBSinduced colitis. Regul Pept. 2007;141:25–34. doi: 10.1016/j.regpep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koetzner L, Grover G, Boulet J, Jacoby HI. Plant-derived polysaccharide supplements inhibit dextran sulfate sodium-induced colitis in the rat. Dig Dis Sci. 2010;55:1278–1285. doi: 10.1007/s10620-009-0848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan YM, Zhu YQ, Xia B, Luo J. Treating TNBS-induced colitis in rats with probiotics. Turk J Gastroenterol. 2011;22:486–493. doi: 10.4318/tjg.2011.0247. [DOI] [PubMed] [Google Scholar]
- 14.Galvez-Llompart M, Recio MC, Garcia-Domenech R. Topological virtual screening: a way to find new compounds active in ulcerative colitis by inhibiting NF-kappaB. Mol Divers. 2011;15:917–926. doi: 10.1007/s11030-011-9323-4. [DOI] [PubMed] [Google Scholar]
- 15.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 16.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67:909–916. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, MacDonald JA. Mitogenactivated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009;75:1031–1041. doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 18.ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507–512. doi: 10.1136/gut.50.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 20.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Tolllike receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 22.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M, Majorova E, Arato A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151:34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J Histochem Cytochem. 2008;56:267–274. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Bokodi G, Vasarhelyi B, Korponay-Szabo IR, Tulassay T, Arato A. Increased mucosal expression of Toll-like receptor (TLR)2 and TLR4 in coeliac disease. J Pediatr Gastroenterol Nutr. 2007;45:187–193. doi: 10.1097/MPG.0b013e318064514a. [DOI] [PubMed] [Google Scholar]