Abstract
Aim: Emodin was found effective in suppressing proliferation of cancer cells including colorectal cancer (CRC), but the mechanisms were still unclear. This study was aimed to investigate the possible mechanism of emodin’s anti-CRC effects. Methods: Two most frequently used CRC cell lines, SW480 and SW620, were investigated in this study. Serially diluted emodin solutions were used to incubate CRC cells. siRNAs were used to silence the expressions of p38 and Puma respectively. Intracellular ROS production was detected by DCFH-DA staining; proliferation and apoptosis of CRC cells were assessed by MTT assay and Hoechst staining respectively. Western blotting was applied to evaluate the activation of p38/p53/Puma signaling. Results: Both in SW480 and SW620 cells, emodin inhibited proliferation by inducing ROS-mediated apoptosis in a concentration-dependent manner. The p38/p53/Puma signaling was also activated after emodin incubation in a concentration-dependent manner. The ROS scavenger NAC, p38 silencing and Puma silencing impaired the anti-proliferation and apoptosis- inducing effects of emodin. Conclusions: emodin inhibited proliferation of human CRC cells by inducing cell apoptosis by activating ROS/p38/p53/Puma signaling.
Keywords: Colorectal cancer, emodin, apoptosis, reactive oxygen species
Introduction
Malignant cancer has become a public health issue which accounts for 1/8 of deaths worldwide. As a common cancer, colorectal cancer (CRC) is the third most frequent cancer in male (right after lung cancer and prostate cancer) and the second frequent cancer in female (after breast cancer) [1]. The occurrence of CRC has been climbing constantly in recent decades around the world, especially in developed and industrialized countries [2]. It was reported that the average 5-year survival rate of CRC was 46.8% for male and 48.4% for female [3]. As the development of medical treatment and early diagnosis, the mortality rate of CRC was significantly lowered in several western developed countries [4] recently, however, its prognosis is still pessimistic. Thus, novel therapeutic agents are significant in CRC treatment.
As the one of the solid malignant tumors, the most effective treatment is surgical resection. However, many patients with CRC were diagnosed after obvious clinical manifestations were noticed when the cancer developed to the advanced stage [5]. Thus, these patients were not appropriate candidates for surgical treatment. The alternative radiotherapies and chemotherapies are narrowed by their limited effectiveness and multiple side effects [6]. Agents extracted from natural plants which are also called phytochemicals showed their effects in cancer treatment and prevention via targeting and regulating multiple molecular pathways [7]. The existence of emodin was found in numerous natural herbs such as Rheum and Polygonam which have a long history in Traditional Chinese Medicine [8]. Also referred as 1,3,8-trihydroxy-6-methyl-anthraquinone, emodin is considered have various biological activities such as anti-inflammation [9], anti-infection [10], immuno-suppression [11] and so on. It was also believed that emodin was a potent anti-cancer agent, showing cytotoxicity against malignant cancers such as gallbladder cancer, liver cancer, cervical cancer and so on [12].
Multiple mechanisms of emodin’s anti-cancer activities were indicated in previous studies. For instance, cell-cycle arrest [13], NF-κB deactivation [14], PI3K/AKT pathway [15] and PKC pathway activation [16]. However, the exact mechanisms are still unclear. Emodin was known to stimulate intracellular reactive oxygen species (ROS) generation which was considered as a typical apoptosis inducer by targeting various pathways. A recent study reported that increased intracellular oxidative stress lead to p38 activation which further resulted in elevation of expression and transcriptional activity of p53 [17]. Additionally, p53 is generally considered as the up-stream molecule which activates Puma (p53-upregulated mediator of apoptosis) [18]. The latter could result in mitochondria dysfunction, cytochrome-c release, caspase cascade activation and apoptosis [19]. Thus, it is reasonable for us to speculate that emodin induces apoptosis of CRC cells through activating p53/p38/Puma pathway by triggering ROS production.
In order to testify our presupposition, this study was implemented. Two types of human CRC cells, namely SW480 and SW620 cells were selected. After incubated with emodin, cell proliferation and apoptosis were investigated. Mechanically, intracellular ROS production and activation of down-stream p53/p38/Puma pathway were also observed. We believe that results from this study would not only provide more solid evidence for potential clinical application of emodin or emodin-contained compounds in CRC treatment, but also improve our understanding of emodin’s anti-cancer pharmacological mechanisms.
Material and methods
Cell culture and treatment
SW480 and SW620 cells which were purchased from China Center for Type Culture Collection (CCTCC, China) were used in this study. SW480 and SW620 cells were cultured in RPMI 1640 medium (Hyclone, USA) which was supplemented with 10% bovine serum (FBS, Hyclone, USA), 1% antibiotic mix (Sigma-Aldrich, USA, containing 150 μmol/L streptomycin and 100 U/ml penicillin) and 2.0 mmol/L L-glutamine (Sigma-Aldrich, USA). Cells were maintained in humidified condition supplied with 95% fresh air and 5% CO2 at 37°C. In some cases, cells were incubated with serial diluted emodin solution ranging from 0 μmol/L to 100 μmol/L for 24 hours. In other cases, emodin-treated cells were treated with N-acetylcysteine (NAC), the typical ROS scavenger, at 10 mmol/L for 24 hours.
RNA interference (RNAi) application
Specific small interference RNA (siRNA) against p38 and Puma were designed and provided by GenePharma (Shanghai, China). The sequence of p38 siRNA was 5’-GUGCCAACCUGAUGCAGCU-3’ (sense) [20]; sequence of Puma siRNA was 5’GAAUGCCAGUGGUCACACG-3’ (sense) [21]. siRNAs (25 mg/dish) were transfected into the cells with the aid of HiperFect transfection kit (Qiagen, USA) according to manufacturer’s instructions. Cells were harvested for subsequent experiments after 48-hour incubation. Targeted gene knock-down was determined by western blotting by using anti-p53 or anti-Puma antibodies.
Cell proliferation assay
Colorimetic 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the proliferation of SW480 and SW620 cells. Cells (1×104/well) were seeded into wells on a 96-well plate and then incubated with MTT solution (5 mg/ml) for 4 hours at 37°C. The absorbance at 540 nm (A540) was detected by plate reader. Finally the inhibition rate was calculated by “[1-A540 (experimental well)/A540 (control well)] ×100%”.
Cell apoptosis determination
The apoptosis of CRC cells was determined by apoptotic indicator, Hoechst, staining. Briefly, cells were harvested and fixed by 4% paraformaldehyde at 37°C for 1 hour. Then, after washed by PBS, cells were stained by 5 μmol/L Hoechst 33342 (Sigma-Aldrich, USA) at 37°C for 30 minutes in a humidified dark chamber. Then a fluorescence microscopy (Nikon, Japan) was used to capture the fluorescent images showing the morphological features. Hoechst- positive cells were considered apoptotic ones.
Intracellular ROS detection
DCFH-DA staining was used to assess the intracellular level of ROS in SW480 and SW620 cells. The cells were incubated with DCFH-DA (10 μmol/L) which was diluted by RPMI 1640 medium at 37°C for 20 minutes. A fluorescence microscopy (Nikon, Japan) was used to capture the fluorescent images which were then analyzed by Image Pro software (Media Cybernetic, USA).
Western blotting
After lyzed by RIPA lysis buffer system (Santa Cruz, USA), the cell lysates of SW480 and SW620 cells were harvested. The total protein was extracted by using Protein Extraction kit (Beyotime, China) while the nuclear protein was isolated by R0050 nuclear protein extraction kit (Solarbio, China) per manufacturer’s instructions. Protein concentration was examined by a BCA kit (Thermo). 45 μg of protein sample was loaded and then separated in a SDS gel by vertical electrophoresis. Separated proteins were then transblotted to poly vinylidene difluoride (PVDF) membranes electronically. After the membranes were incubated by 5% defatted milk to eliminate non-specific bindings. Then specific antibodies against p38, phosphorylated p38 (p-p38), p53, Puma and caspase-3 were used to detect the expression of targeted proteins. GAPHD and Histone were introduced as internal references for western blotting analysis of total protein and nuclear protein. Immunoblots were then detected by SuperSignal West Pico kit (Peirce).
Statistics
Results in this study were presented as (mean ± SD) which were then analyzed by software SPSS (SPSS, USA). Differences between groups were analyzed by one-way ANOVA or student’s t-tests. P<0.05 was considered statistically significant.
Results
Emodin incubation inhibited proliferation of CRC cells which was reversed by NAC, p38 silence or Puma silence.
As shown in Figure 1, after incubated with emodin, the proliferation of CRC cells was inhibited significantly in a concentration-dependent manner. However, also demonstrated in Figure 1, NAC incubation, p38 or Puma silence significantly impaired emodin’s anti-proliferation effects against cultured CRC cells.
Figure 1.
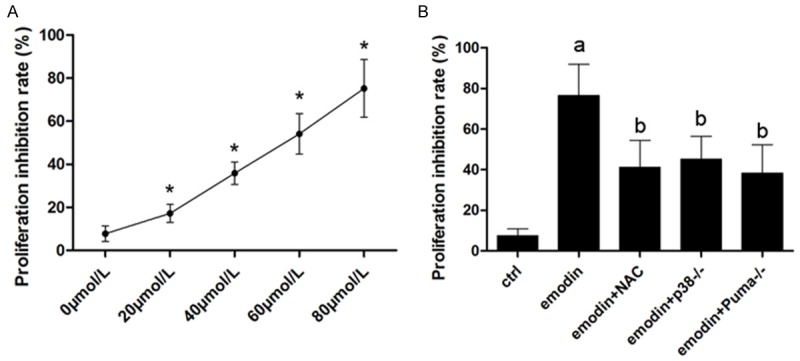
Affections of NAC, p38 silencing and Puma silencing on emodin’s anti-proliferation effects. A. After incubated with serially diluted emodin solutions, namely 0, 20, 40, 60 and 80 μmol/L, the proliferation inhibition rate of SW480 cells were detected by MTT assay. [*differences were significant when compared with previous concentration]. B. After incubated with emodin solution (concentration at 80 μmol/L), SW480 cells were treated by NAC or p38 siRNA or Puma siRNA. Columns in this figure demonstrated the proliferation inhibition rate assessed by MTT assay. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “emodin”]. Similar results were found in SW620 cells which were not demonstrated in this figure.
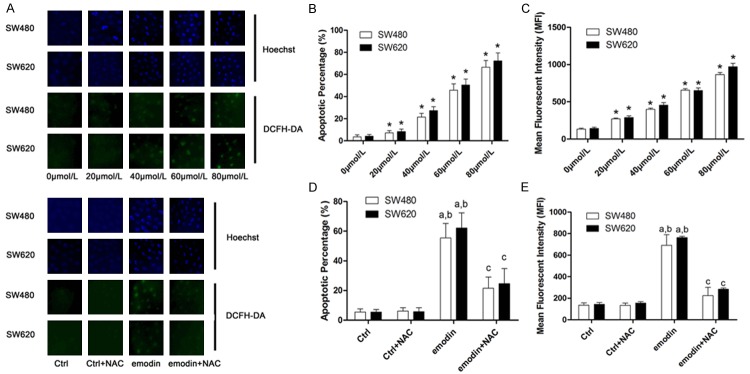
NAC incubation significantly attenuated emodin-induced ROS-mediated apoptosis in SW480 and SW690 CRC cells
As demonstrated in Figure 2, intracellular ROS production was elevated after emodin incubation in a concentration-dependent manner. NAC was used to treat CRC cells after emodin incubation. Detected by Hoechst staining, the emodin-induced apoptosis of CRC cells was also in a concentration-dependent manner, which was attenuated by NAC treatment.
Figure 2.
Affections of NAC on emodin-induced ROS production and apoptosis in SW480 and SW620 cells. A. Demonstrated the fluorescent images of Hoechst and DCFH-DA staining. Hoechst staining is used to mark the apoptotic cells while DCFH-DA staining is considered a reliable method to detect intracellular ROS production. The upper part demonstrated Hoechst and DCFH-DA staining of SW480 and SW620 cells incubated with serially diluted emodin solutions; the lower part showed the Hoechst and DCFH-DA staining of SW480 and SW620 cells received NAC and/or emodin treatments. B. Columns demonstrated the apoptotic percentage of SW480 and SW620 cells incubated with serially diluted emodin solutions. [*differences were significant when compared with previous concentration]. C. Columns demonstrated the mean fluorescent intensity of DCFH-DA staining in SW480 and SW620 cells incubated with serially diluted emodin solutions. [*differences were significant when compared with previous concentration]. D. Columns demonstrated the apoptotic percentage of SW480 and SW620 cells received NAC and/or emodin treatments. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “Ctrl+NAC”; c. differences were significant when compared with “emodin”]. E. Columns showed the mean fluorescent intensity of DCFH-DA staining in SW480 and SW620 cells received NAC and/or emodin treatments. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “Ctrl+NAC”; c. differences were significant when compared with “emodin”].
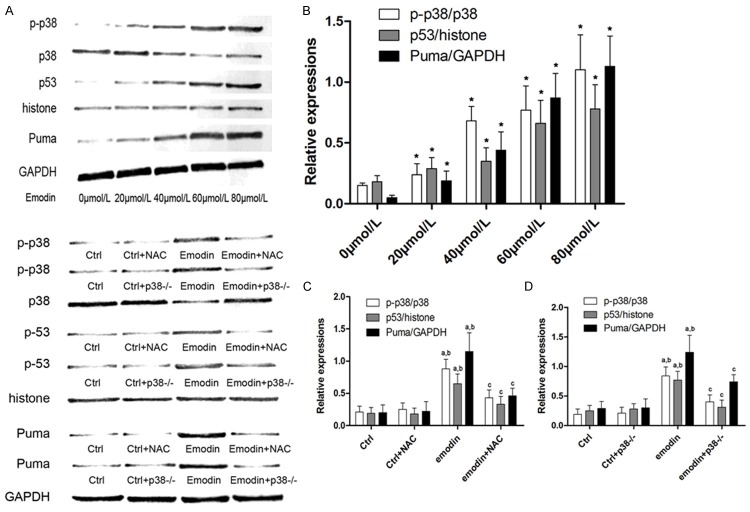
ROS/p38/p53/Puma signaling pathway was activated by emodin incubation in SW480 and SW690 CRC cells
Figure 3 showed the activation of ROS/p38/p53/Puma signaling pathway in emodin incubated CRC cells. After NAC treatment, p38 phosphorylation, p53 unclear translocation and Puma expression levels decreased significantly. In p38 silenced cells, although ROS generation was not affected, the p53 unclear translocation and subsequent Puma expression level were inhibited. These results indicated that ROS/p38/p53/Puma formed a signaling pathway and activated after emodin incubation.
Figure 3.
Affections of NAC and p38 silencing on emodin›s effects on p-38 phosphorylation, p53 unclear translocation and Puma expression levels. A. The upper part demonstrated the immunoblots of phosphorylated p38, total p38, p53, histone, Puma and GAPDH in SW480 cells incubated with serially diluted emodin solutions. The lower part showed the immunoblots of phosphorylated p38, total p38, p53, histone, Puma and GAPDH in SW480 cells treated by emodin and p38 siRNA and/or emodin respectively. B. Columns demonstrated the p-38 phosphorylation level (p-p38/p38), p53 unclear translocation (p53/histone) and Puma expression (Puma/GAPDH) in SW480 cells incubated with serially diluted emodin solutions. [*differences were significant when compared with previous concentration]. C. Columns showed the p-38 phosphorylation level (p-p38/p38), p53 unclear translocation (p53/histone) and Puma expression (Puma/GAPDH) in SW480 cells treated by NAC and/or emodin solutions. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “Ctrl+NAC”; c. differences were significant when compared with “emodin”]; D. Columns showed the p38 phosphorylation level (p-p38/p38), p53 unclear translocation (p53/histone) and Puma expression (Puma/GAPDH) in SW480 cells treated by p38 siRNA and/or emodin solutions. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “Ctrl+p38-/-”; c. differences were significant when compared with “emodin”]. Similar results were found in SW620 cells which were not demonstrated in this figure.
Puma silencing significantly relieved emodin- induced cell apoptosis of SW480 and SW690 CRC cells
Figure 4 demonstrated the effects of Puma silencing on emodin- induced CRC cell apoptosis. Puma silencing abated emodin-induced CRC cell apoptosis which was indicated by Hoechst staining. Mean while, Puma silencing didn’t affect ROS production, p38 phosphorylation and p53 nuclear translocation level in emodin-incubated CRC cells.
Figure 4.
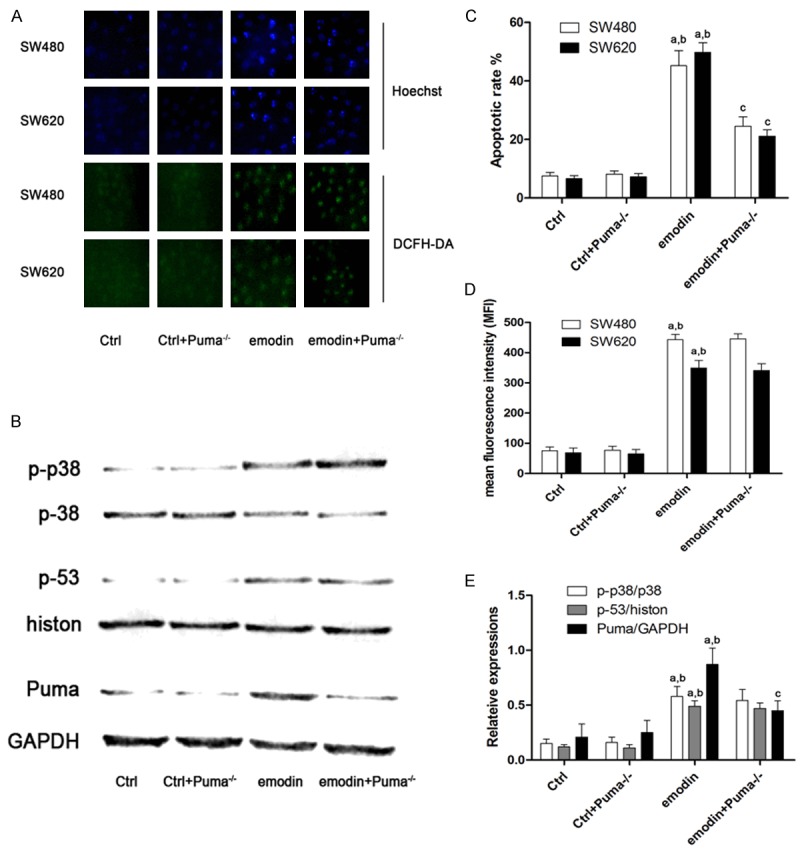
Effects of Puma silencing on emodin-induced ROS production, apoptosis and p38/p53/Puma signaling. A. Demonstrated the captured fluorescent images of Hoechst and DCFH-DA staining on SW480 and SW620 cells received Puma siRNA and/or emodin treatments. B. Demonstrated the immunoblots of phosphorylated p38, total p38, p53, histone, Puma and GAPDH in SW480 cells received Puma siRNA and/or emodin treatments. C. Columns demonstrated the apoptotic rate of SW480 and SW620 cells received Puma siRNA and/or emodin treatments; D. Columns demonstrated the mean fluorescent intensity of DCFH-DA staining of SW480 and SW620 cells received Puma siRNA and/or emodin treatments; E. Columns demonstrated the p-38 phosphorylation level (p-p38/p38), p53 unclear translocation (p53/histone) and Puma expression (Puma/GAPDH) in SW480 cells received Puma siRNA and/or emodin treatments. [a. differences were significant when compared with “Ctrl”; b. differences were significant when compared with “Ctrl+Puma-/-”; c. differences were significant when compared with “emodin”]. The results immunoblots of phosphorylated p38, total p38, p53, histone, Puma and GAPDH in SW620 cells were not shown in this figure which were similar to results in SW480 cells.
Discussion
In recent decades, the incidence of CRC is growing globally. Although the medical technology is rapidly improving, the mortality of CRC is still contributing to a sizeable proportion of cancer-related death in patients with malignant tumors [22]. Currently, surgical resection, radiotherapy and chemotherapy are thought effective in treating CRC, but their clinical applications are restricted due to innate limitations. In recent years, natural products aroused much attention in cancer treatment because of the strong efficacy and mild side effects [23]. Emodin was found effective in suppressing cancer proliferation, invasion and metastasis in cervical cancer, hepatic cancer, gastric cancer and so on. Few investigations, though, reported emodin’s anti-cancer activity and related mechanisms in CRC. In the current study, we reported that emodin inhibited proliferation of CRC cells by inducing apoptosis in a concentration-dependent manner. Furthermore, we testified the possible involvement of ROS/p38/p53/Puma signaling pathway in emodin-induced CRC cell death.
Generally accepted, type of cell death could be divided into necrosis and apoptosis. Also called programmed cell death (PCD), apoptosis is a highly regulated and organized biological process maintaining internal homeostasis by eliminating targeted cells [24]. This process could kill malignant cells without damaging surrounding normal functional cells. Apoptosis is considered one of the key mechanisms of ideal anti-cancer agents [25]. In this study, we found that emodin inhibited the proliferative ability of CRC cells in vitro in a concentration-dependent manner. We further detected apoptosis of CRC after emodin incubation and found that the apoptotic rate was elevated by emodin in a concentration-dependent manner. The results indicated that apoptosis induction was involved in the anti-proliferative effect of emodin against CRC cells.
Excessive intracellular oxidative stress is one of the most important apoptosis-inducing factors which is correlated with apoptotic pathways. Emodin is a natural product extracted from barks and roots of various plants [26]. The molecular structure of emodin is similar to 2,3-dimethoxy-1,4-naphthoquinone which shows the property of quinones, generating ROS when electrons are transferred [27]. Thus, emodin could also recognized as a intracellular ROS generator. In this study, we found that emodin incubation significantly elevated intracellular ROS level in CRC cells. Furthermore, NAC was used to reduce the intracellular ROS generated by emodin in CRC cells. As a result, apoptosis was significantly inhibited. This result suggested that emodin-induced excessive ROS generation induced apoptosis of CRC cells. Thus, a association between emodin’s anti-cancer effect and ROS production activity was established.
p38 is a protein kinase belongs to mitogen- activated protein kinases (MAPK) family, response to various cellular stressful conditions [28]. It is believed that p38 also plays a role in redox signaling transduction [29]. p38 is considered as a sensor of oxidative stress because p38 could be activated under stimulation of excessive intracellular ROS. p38 is phosphorylated to reach activation [30]. Then activated p38 regulates its down-stream transcription factors including p53 [31] which is also regarded as a redox sensor. According to previous studies, as one of the direct down- stream effectors of p38, p53 could be activated by p38 to accumulate in nucleus [32]. Then the expressions of p53’s target genes such as Bak, Noxa, Apaf and Puma are subsequently regulated [33]. In the current study, either ROS reduction or p38 silencing inhibited p53- induced Puma expression, thus, the activation of ROS/p38/p53/Puma pathway was confirmed in emodin-incubated CRC cells.
Puma is a pro-apoptotic protein regulating mitochondria-mediated apoptotic pathway [34]. Mitochondrial dysfunction is induced by several multi-domain proteins such as Bax and Bak which are activated by Puma. Then mitochondrial apoptogenic proteins such as cytochrome c are released from mitochondria to trigger activation of caspase activation which would eventually lead to irreversible cell apoptosis [35]. In this study, after Puma was silenced by RNAi technique, the emodin-induced apoptosis of CRC cells decreased significantly. These results indicated that the apoptotic signaling transduction via ROS/p38/p53/Puma pathway in emodin-incubated CRC cells.
In summary, this study demonstrated that emodin inhibited proliferation of human CRC cells by inducing cell apoptosis. Moreover, our study suggested excessive intracellular ROS generation was the initiator of emodin- induced CRC apoptosis. Mechanically, the apoptotic signaling is transduced through p38/p53/Puma signaling pathway. These results not only deepened our understanding of mechanisms of emodin’s anti-cancer activity, but also augmented solid evidences for future clinical application of emodin-associated drugs in human CRC treatment.
Disclosure of conflict of interest
None.
References
- 1.Tarraga Lopez PJ, Albero JS, Rodriguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol. 2014;7:33–46. doi: 10.4137/CGast.S14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshihara M, Hiyama T, Tanaka S. [Epidemiology of colorectal cancer] . Nihon Naika Gakkai Zasshi. 2007;96:200–206. doi: 10.2169/naika.96.200. [DOI] [PubMed] [Google Scholar]
- 3.Bendardaf R. Colorectal cancer: from epidemiology to current treatment. Libyan J Med. 2006;1:42–59. doi: 10.4176/060714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yee YK, Tan VP, Chan P, Hung IF, Pang R, Wong BC. Epidemiology of colorectal cancer in Asia. J Gastroenterol Hepatol. 2009;24:1810–1816. doi: 10.1111/j.1440-1746.2009.06138.x. [DOI] [PubMed] [Google Scholar]
- 5.Cartwright TH. Treatment decisions after diagnosis of metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:155–166. doi: 10.1016/j.clcc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Sharma RA, Wasan HS, Love SB, Dutton S, Stokes JC, Smith JL. FOXFIRE: a phase III clinical trial of chemo-radio-embolisation as first-line treatment of liver metastases in patients with colorectal cancer. Clin Oncol (R Coll Radiol) 2008;20:261–263. doi: 10.1016/j.clon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review) J Nutr Biochem. 2003;14:64–73. doi: 10.1016/s0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 8.He L, Bi JJ, Guo Q, Yu Y, Ye XF. Effects of emodin extracted from Chinese herbs on proliferation of non-small cell lung cancer and underlying mechanisms. Asian Pac J Cancer Prev. 2012;13:1505–1510. doi: 10.7314/apjcp.2012.13.4.1505. [DOI] [PubMed] [Google Scholar]
- 9.Alisi A, Pastore A, Ceccarelli S, Panera N, Gnani D, Bruscalupi G, Massimi M, Tozzi G, Piemonte F, Nobili V. Emodin prevents intrahepatic fat accumulation, inflammation and redox status imbalance during diet-induced hepatosteatosis in rats. Int J Mol Sci. 2012;13:2276–2289. doi: 10.3390/ijms13022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JR, Oh DR, Cha MH, Pyo BS, Rhee JH, Choy HE, Oh WK, Kim YR. Protective effect of polygoni cuspidati radix and emodin on Vibrio vulnificus cytotoxicity and infection. J Microbiol. 2008;46:737–743. doi: 10.1007/s12275-008-0232-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YY, Liu B, Ge XP, Liu WB, Xie J, Ren M, Cui YT, Xia SL, Chen R, Zhou Q, Pan L, Yu Y. The influence of various feeding patterns of emodin on growth, non-specific immune responses, and disease resistance to Aeromonas hydrophila in juvenile Wuchang bream (Megalobrama amblycephala) Fish Shellfish Immunol. 2014;36:187–193. doi: 10.1016/j.fsi.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y. Chemosensitization by emodin, a plant-derived anti-cancer agent: mechanism of action. Cancer Biol Ther. 2008;7:476–478. doi: 10.4161/cbt.7.3.5584. [DOI] [PubMed] [Google Scholar]
- 13.Narender T, Sukanya P, Sharma K, Bathula SR. Preparation of novel antiproliferative emodin derivatives and studies on their cell cycle arrest, caspase dependent apoptosis and DNA binding interaction. Phytomedicine. 2013;20:890–896. doi: 10.1016/j.phymed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Lin ML, Lu YC, Chung JG, Wang SG, Lin HT, Kang SE, Tang CH, Ko JL, Chen SS. Down-regulation of MMP-2 through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol Carcinog. 2010;49:783–797. doi: 10.1002/mc.20652. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim CM, Choi YW, Lee YC. Emodin induces neurite outgrowth through PI3K/Akt/GSK-3beta-mediated signaling pathways in Neuro2a cells. Neurosci Lett. 2015;588:101–107. doi: 10.1016/j.neulet.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Acevedo-Duncan M, Russell C, Patel S, Patel R. Aloe-emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int Immunopharmacol. 2004;4:1775–1784. doi: 10.1016/j.intimp.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Li X, Li Y, Li N, Shi X, Ding H, Zhang Y, Liu G, Wang Z. Non-esterified fatty acids activate the ROS-p38-p53/Nrf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis. 2014;19:984–997. doi: 10.1007/s10495-014-0982-3. [DOI] [PubMed] [Google Scholar]
- 18.Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci U S A. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chipuk JE, Green DR. PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle. 2009;8:2692–2696. doi: 10.4161/cc.8.17.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X, Li L, Zhang L, Wu J, Zhou Y, Zhao Y, Zhao J. Inhibition of thioredoxin-1 with siRNA exacerbates apoptosis by activating the ASK1-JNK/p38 pathway in brain of a stroke model rats. Brain Res. 2015;1599:20–31. doi: 10.1016/j.brainres.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Tian M, Jin L, Jia H, Jin Y. PUMA is invovled in ischemia/reperfusion-induced apoptosis of mouse cerebral astrocytes. Neuroscience. 2015;284:824–832. doi: 10.1016/j.neuroscience.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Rozen P, Liphshitz I, Barchana M. The changing epidemiology of colorectal cancer and its relevance for adapting screening guidelines and methods. Eur J Cancer Prev. 2011;20:46–53. doi: 10.1097/CEJ.0b013e328341e309. [DOI] [PubMed] [Google Scholar]
- 23.Basmadjian C, Zhao Q, Bentouhami E, Djehal A, Nebigil CG, Johnson RA, Serova M, de Gramont A, Faivre S, Raymond E, Desaubry LG. Cancer wars: natural products strike back. Front Chem. 2014;2:20. doi: 10.3389/fchem.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Sun B, Gao Y, Meng QH, Jiang HC. An experimental study of emodin assisted early enteral nutrition for the treatment of severe acute pancreatitis. Hepatogastroenterology. 2008;55:33–40. [PubMed] [Google Scholar]
- 27.Qu K, Shen NY, Xu XS, Su HB, Wei JC, Tai MH, Meng FD, Zhou L, Zhang YL, Liu C. Emodin induces human T cell apoptosis in vitro by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacol Sin. 2013;34:1217–1228. doi: 10.1038/aps.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Wang Q, Long Y, Zhang R, Wei X, Xing M, Gu H, Xie X. Stress-mediated p38 activation promotes somatic cell reprogramming. Cell Res. 2013;23:131–141. doi: 10.1038/cr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riazantseva NV, Novitskii VV, Kaigorodova EV, Chasovskikh N, Starikova EG. [Mitogenactivated protein kinases JNK and p38 as redox-dependent molecular targets correction of programmed cell death disturbances in oxidative stress condition] . Usp Fiziol Nauk. 2009;40:3–11. [PubMed] [Google Scholar]
- 30.Gaffey K, Reynolds S, Plumb J, Kaur M, Singh D. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42:28–41. doi: 10.1183/09031936.00170711. [DOI] [PubMed] [Google Scholar]
- 31.Cardaci S, Filomeni G, Rotilio G, Ciriolo MR. p38(MAPK)/p53 signalling axis mediates neuronal apoptosis in response to tetrahydrobiopterin-induced oxidative stress and glucose uptake inhibition: implication for neurodegeneration. Biochem J. 2010;430:439–451. doi: 10.1042/BJ20100503. [DOI] [PubMed] [Google Scholar]
- 32.Gaitonde SV, Riley JR, Qiao D, Martinez JD. Conformational phenotype of p53 is linked to nuclear translocation. Oncogene. 2000;19:4042–4049. doi: 10.1038/sj.onc.1203756. [DOI] [PubMed] [Google Scholar]
- 33.Gotz C, Montenarh M. P53 and its implication in apoptosis (review) Int J Oncol. 1995;6:1129–1135. doi: 10.3892/ijo.6.5.1129. [DOI] [PubMed] [Google Scholar]
- 34.Vousden KH. Apoptosis. p53 and PUMA: a deadly duo. Science. 2005;309:1685–1686. doi: 10.1126/science.1118232. [DOI] [PubMed] [Google Scholar]
- 35.Cheng WP, Wang BW, Chen SC, Chang H, Shyu KG. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc Res. 2012;93:181–189. doi: 10.1093/cvr/cvr280. [DOI] [PubMed] [Google Scholar]