Abstract
Background: Current diagnostic procedures of cancers are invasive and non-specific. MicroRNAs (miRNAs) have become promising molecular markers for gastric cancer (GC) predication. However, there have been inconsistencies in the literature regarding the suitability of circulating miRNAs for early detection of cancers. Methods: We performed a comprehensive meta-analysis to integrate an evaluation index for diagnostic accuracy of miR-223 in diagnosing cancer patients. Furthermore, we conducted an independent validation set of 50 gastric cancer patients and 50 healthy controls comparing miR-223 expression. We also analyzed miR-223 expression in vitro. Results: A total of 11 studies met the inclusion criteria and therefore included in this meta-analysis. We found that miR-223 yielded a pooled area under ROC curve (AUC) of 0.89 (sensitivity: 81%, specificity: 84%) in discriminating cancer from controls. In our validation test, plasma miR-223 levels in GC patients were significantly higher than that in healthy controls (P<0.01). ROC curve analysis showed that AUC was 0.812 with a sensitivity of 70% and specificity of 80%. Moreover, the expression trend of miR-223 in plasma samples was in accordance with that of tissue and cell samples. Conclusion: Current evidences suggested that plasma miR-223 could be a reliable and non-invasive biomarker for cancer diagnosis. Further large-scale prospective studies are necessary to validate their potential applicability in human cancer diagnosis.
Keywords: miR-223, cancer, meta-analysis, diagnosis, biomarker
Introduction
Recently, there are a significant percentage of patients who suffered from various types of cancers. However, the prognosis of the majority of them is poor because the cancers are usually diagnosed at advanced stages, which, unfortunately, indicates that they have almost missed the optimal treatment at the early stage. Therefore, one of the biggest challenges in cancer treatment is the lack of sensitive and specific biomarker identification for early cancer detection [2,3]. Although endoscopy has been widely used in the clinics, it still has limitations for its invasive nature and relatively high costs [4].
It is considered that cancer-related biomarkers in blood would be quite helpful in early cancer diagnosis and tumor progression monitoring [5]. As non-invasive methods for cancer diagnosis, some of the currently available circulating biomarkers, such as CEA, pepsinogen (PG) I/II, progesterone receptor (PR) and estrogen receptor (ER) are being used without performing any biopsy or surgical procedure. Nevertheless, these tests usually present low sensitivity and specificity [6]. Therefore, there is an urgent need for discovering novel non-invasive biomarkers with higher sensitivity in order to improve the diagnostic accuracy for cancers.
MicroRNAs (miRNAs) are a class of evolutionarily conserved and 22nt non-coding RNA molecules that regulate a variety of critical cellular processes, including cell growth, differentiation, proliferation, apoptosis and metabolism [7]. The ectopic expressions of miRNAs, along with their profiles in human cancers, could be applied not only in cancer prediction and prognosis but also in tumor classification and progression [8]. Besides, recent studies have identified that tumor-associated RNAs, especially miRNAs, were readily detectable in circulating body fluids from cancer patients [9]. Expression levels of MiR-223 were found to be significantly higher in various types of tumor tissues. In 2010, Zhang et al [10] first reported that miR-223 was significantly up-regulated in plasma of esophageal squamous cell carcinoma (ESCC) patients compared to healthy individuals, suggesting that miR-223 could be a potential non-invasive molecule for ESCC screening. From then on, an increasing number of researches have emerged regarding the clinical value of miR-223 in cancers [11-20].
To comprehensively understand whether miR-223 could serve as a diagnostic biomarker for cancers, we performed a systematic meta-analysis to evaluate the diagnostic efficiency of circulating miR-223 in cancer patients from published literatures, combined with a validation study, and to identify a novel non-invasive biomarker for early cancer detection.
Materials and methods
Search strategy
This meta-analysis was conducted according to the guidelines of diagnostic meta-analysis. Eligible studies published up to April 13, 2015 were selected for meta-analysis by conducting asystematic literature search of public databases including PubMed and Embase. No restriction was used on language, year of publication or publishing status. The keywords employed for literature retrieval included: “circulating” or “serum” or “plasma”, “miRNA-223” or “microRNA-223” or “miR-223”, and “cancer” or “carcinoma” or “neoplasm”. In addition, reference lists of eligible articles were independently searched manually to obtain additional sources.
Selection of publications
All the studies were carefully reviewed by two investigators (Z.X.Y and J.G.P) independently based on titles and abstracts, and then found full text for any potential eligibility. Any disagreement was resolved by fully discussion to consensus. Furthermore, if necessary, we asked the original authors for missing data. All publications included in the meta-analysis met the following criteria: (1) Patients with any type of cancers, and identified by the diagnosis of histopathological confirmation; (2) All blood samples were collected prior to any treatments; (3) Studies detecting the expression levels of circulating miRNAs and investigating their associations with cancer diagnosis were included; (4) Studies should contain the data of sensitivity, specificity (or the possibility of deriving such values from the data); (5) Only the study enrolled more than 20 patients and matched controls were included. Studies were excluded if they got any of the following items: (1) Duplicate study; (2) Letters, editorials, meeting abstracts, case reports and reviews; (3) Unqualified patients and control subjects, as well as their blood samples; (4) Studies with missing data. If the same author reported their results acquired from the overlapping population, only the nearest or the most complete study was included.
Data extraction and quality assessment
The following patients’ characteristics were collected for each study: author name, publication year, country and ethnicity, sample type, normalization control, sample size and data for two-by-two tables (sensitivity and specificity). The Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist was used to systematically assess the quality of the articles included in the diagnostic meta-analysis. Specifically, 14 items from the QUADAS checklist were applied to each article, and an answer of “Yes”, “No” or “Unclear” and only “Yes” would result in a score.
Validation of miR-223 expression in plasma, tissues and cells
The expression levels of miR-223 were measured in 50 pairs of plasma samples from gastric cancer patients and controls using qRT-PCR analysis. Plasma samples were collected from First Affiliated Hospital of Nanjing Medical University prior to any treatments with written consent. Tissue samples were collected after surgery. Extraction of miRNA from plasma and tissues was used by Trizol (Takara, Japan) with miRNeasy Mini kits (Qiagen, Valencia, CA), and then reverse-transcribed to cDNA. We quantified miRNA expression to U6 using the 2-ΔCt method. The study was approved by the institutional review board of Nanjing Medical University.
An immortalized human gastric epithelial cell line GES-1 was cultured in RPMI 1640 (gibico, USA) medium supplemented with 10% fetal bovine serum (FBS), as described previously. The human GC cell lines MKN45 and 7901 were cultured in RPMI-1640 (Hyclone, USA).
Statistical analysis
All statistical analyses were performed by STATA 13.0 statistical software (Stata Corporation, TX, USA). All data from each study (true positives, false positives, true negatives and false negatives) were extracted to obtain pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic score (DS), diagnostic odds ratio (DOR) and their 95% confidence interval (CI), the summary receiver operator characteristic (SROC) curve and calculate the area under the curve (AUC). The Spearman correlation coefficient was used to evaluate cut-off threshold effects between sensitivity and specificity. In addition to a P value less than 0.05, heterogeneity across studies was assessed using Cochran’s Q and I2 statistics; I2 more than 50% indicated the existence of significant heterogeneity. Meta-regression was performed to explore the possible heterogeneity. DerSimonian and Laird’s random-effects model was applied when heterogeneity existed; otherwise, the fixed-effects model using the Mantel-Haenszel method was employed. The presence of publication bias was detected using the Deek’s funnel plot asymmetry test; a P value less than 0.10 was considered statistically significant. Differences in distributions of demographic characteristics and plasma miRNA expression levels between GC and controls, in validation tests, were evaluated with the Student’s t test and Pearson’s χ2 test. Then, we performed ROC curves analysis and calculated AUCs to evaluate the associations of miR-223 and GC by SPSS 18.0 (CA, USA). A P value less than 0.05 for two-tailed was considered statistically significant.
Results
Literature search and study characteristics
The procedure of study selection was presented in Figure 1. A total of 176 relevant articles were retrieved form a primary literature search. Thirty articles with information on GC diagnosis and miR-223 remained after series of exclusion criteria were applied (e.g. review or letters, title and abstract screening, etc.). Another 19 articles were excluded as lack of sufficient data for diagnostic analyses. Eleven articles remained [10-20]. The main characteristics of each study are summarized in Table 1. There were a total of 953 patients and733 controls. 10 studies investigated Asian populations and one study investigated Caucasians; the studies had serum (n=8), plasma (n=2) or bone marrow (n=1) samples. All enrolled studies utilized qRT-PCR with SYBR assay to measure miR-223 expression. The quality of the articles was assessed according to QUADAS (Table S1). The majority of included studies in this meta-analysis fulfilled 11 or more of the 14 items in QUADAS, indicating that the overall quality of included studies is good.
Figure 1.
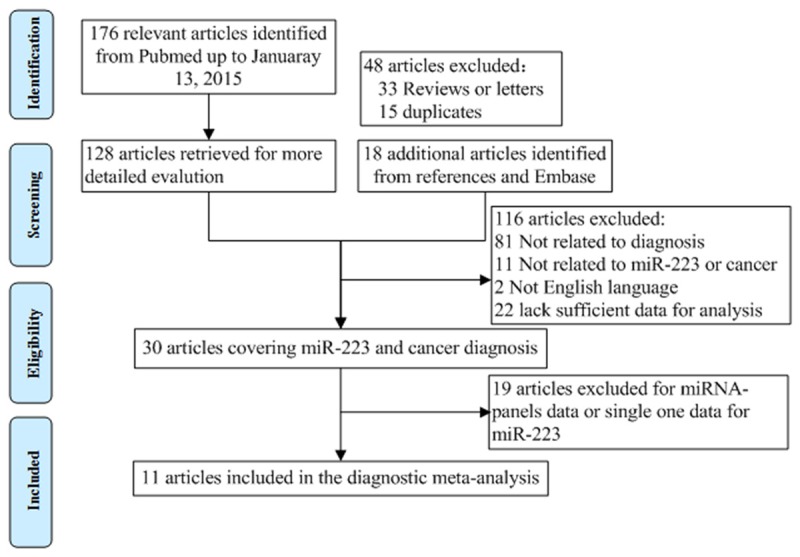
Flow diagram of study selection process.
Table 1.
Characteristics of 11 articles included in our study that reported on using miR-223 as diagnostic biomarkers of various cancers
| Author/Year | Country/ethnicity | Sample | Cancer type | Case/control | AUC | Sensitivity | Specificity | Quality score (QUADAS) |
|---|---|---|---|---|---|---|---|---|
| Zhang/2010 | China/Asian | serum | ESCC | 149/100 | 0.911 | 83.2% | 83.0% | 12 |
| Zhu/2011 | China/Asian | bone marrow | Leukemia | 147/147 | 0.853 | 76.62% | 90.0% | 12 |
| Li/2012 | China/Asian | plasma | GC | 70/70 | 0.9089 | 84.29% | 88.57% | 12 |
| Xu/2012 | China/Asian | serum | HCC | 101/89 | 73.55% | 85.54% | 12 | |
| Sara/2012 | Canada/Caucasian | serum | OC | 30/26 | 0.81 | 96.2% | 60.0% | 12 |
| Jia/2013 | China/Asian | serum | EEC | 26/22 | 0.727 | 57.89% | 95.08% | 12 |
| Kim/2013 | Korea/Asian | serum | GC | 31/15 | 0.750 | 80.11% | 69.04% | 11 |
| Geng/2014 | China/Asian | plasma | NSCLC | 126/60 | 0.96 | 87.0% | 86.0% | 12 |
| Zheng/2014 | China/Asian | serum | CRC | 160/94 | 0.890 | 83.32% | 84.57% | 12 |
| Wang/2014 | China/Asian | serum | GC | 50/47 | 0.85 | 81.0% | 78.0% | 12 |
| Wu/2014 | China/Asian | serum | ESCC | 63/63 | 0.772 | 63.13% | 81.11% | 12 |
Diagnostic accuracy of circulating miR-223 in discriminating cancers
Table 2 illustrates the pooled results of miR-223 in various cancers. The overall analysis of all cancers showed that circulating miR-223 has a relatively good diagnostic performance in cancers, with sensitivity of 0.81 (95% CI: 0.75-0.86), specificity of 0.84 (95% CI: 0.80-0.88) (Figure 2), AUC of 0.89 (0.86-0.92) and DOR of 22 (95% CI: 16-31) (Figure S1A). Since likelihood ratios (LRs) are considered to be more comprehensive and steady diagnostic values of screening tests, we calculated PLR and NLR to predict the diagnostic performance of circulating miR-223. We observed that the pooled PLR and NLR were 5.1 (95% CI: 4.1-6.3) and 0.23 (95% CI: 0.17-0.30) (Figure S1B). The HSROC curves illustrated the estimates of sensitivity and specificity of the eligible studies, in which the summary point was located near the upper left corner of the HSROC curve, and the beta was -0.44 with a P value of 0.461, indicating symmetry of the HSROC curve (Figure S2). Besides, the lambda was 3.24 (95% CI: 2.55-3.91), indicating relatively high accuracy to distinguish GC cases from healthy controls.
Table 2.
Summary sensitivity, specificity, DOR, DS, PLR and NLR of circulating miR-223 for diagnosing various cancers
| Variables | Pooled | I2 a (%) | P a value |
|---|---|---|---|
| Sensitivity | 0.81 (0.75-0.86) | 71 (53.17-88.83) | 0.00 |
| Specificity | 0.84 (0.80-0.88) | 54.95 (24.40-85.51) | 0.01 |
| DOR | 22.34 (16.16-30.90) | 98.36 (97.92-98.79) | 0.00 |
| DS | 3.11 (2.78-3.43) | 34.99 (0.00-81.19) | 0.12 |
| PLR | 5.10 (4.11-6.33) | 16.40 (16.40-84.66) | 0.03 |
| NLR | 0.23 (0.17-0.30) | 68.40 (48.57-88.24) | 0.00 |
I2 and P for heterogeneity test;
DOR, diagnostic odds ratio; DS, diagnostic score; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Figure 2.
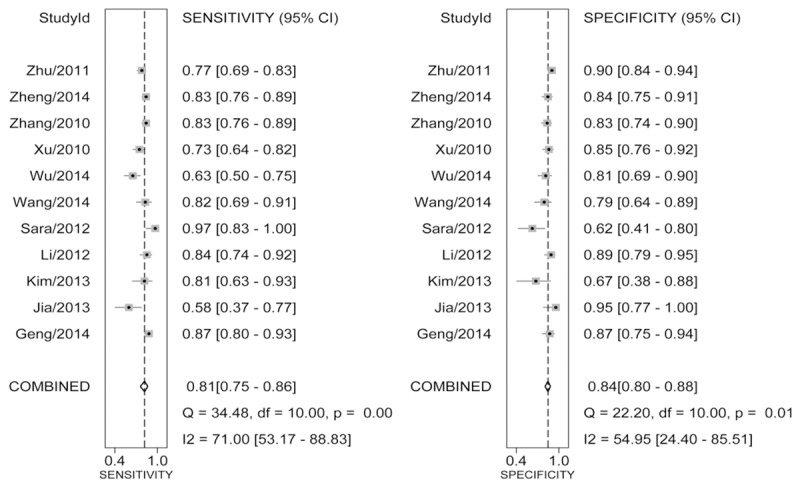
Forest plots for pooled results for diagnosing cancer in circulating miR-223 for sensitivity and specificity and their 95% CI, respectively.
Test of heterogeneity
Heterogeneity might come from either threshold effect or non-threshold effect. The threshold effect was the main cause of heterogeneity, which occurred due to differences in sensitivity/specificity and cut-off value. The common approach to estimate threshold effect has been to use Spearman correlation coefficient of logarithm sensitivity and 1-specificity. In this meta-analysis, we did not find heterogeneity as a result of threshold effect; the Spearman correlation coefficient was 0.309 with P=0.355. The I2 value for heterogeneity analysis was 79%, representing considerable heterogeneity in our meta-analyses. Then, we searched the following sources for heterogeneity: ethnicity, sample type, normalization control and cancer type. Through meta-regression analysis, we found that normalization control, cancer type and ethnicity were the possible major sources of heterogeneity in our study (Table S2).
Publication bias
To assess publication bias of included studies, the Deek’s funnel plot asymmetry test was conducted. The slope coefficient was associated with a P value of 0.574, suggesting a low likelihood of publication bias in our meta-analysis (Figure S3).
Validation of circulating miR-223 in diagnosing GC
To validate whether the reported circulating miR-223 have potential roles in diagnosing cancers, we compared miR-223 expression in GC plasma samples in a case-control study. Basic characteristics were summarized in Table S3. There were no statistically differences in age, sex, smoking status, drinking status or family history of cancer between GC cases and healthy controls (p>0.05 for all). Furthermore, 62% of GC patients were stage III or IV and 40% of the patients were with metastatic status. The expression of miR-223 in plasma was significantly increased in cases compared with that in controls (P<0.01) (Figure 3A). We then performed ROC curve analysis to estimate whether miR-223 could be used as potential diagnostic marker for GC. The AUC of miR-223 was 0.812 (95% CI: 0.730-0.895). At the cut-off values of 0.0813 for miR-223, the sensitivity and specificity were 0.70 and 0.80 (Figure 3B). Besides, as shown in Figure 4A, we found that miR-223 was significantly increased in GC tissues compared with controls (P<0.01), which was in accordance with results in plasma. Furthermore, miR-223 expressed significantly higher in GC cells compared with immortalized GES-1 cells (Figure 4B). Overall, the results suggested that miR-223 could be considered as a diagnostic marker to distinguish cancers from controls.
Figure 3.
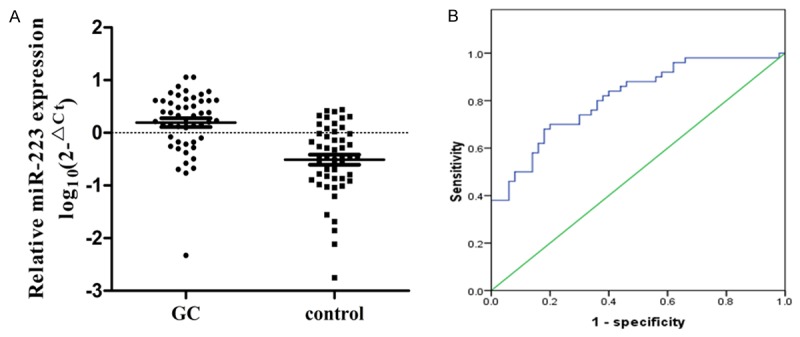
A. Expression level of miR-223 in the plasma of gastric cancer patients was significantly higher compared with controls by qRT-PCR analysis in the validation study; B. ROC curve of the plasma miR-223 for discriminating GC patients from healthy controls.
Figure 4.
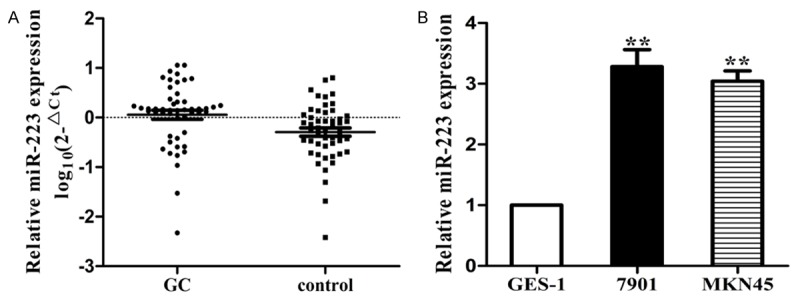
Expression level of miR-223 in tissues and cell lines. A. The expression of miR-223 was significantly higher in GC tissues compared with controls; B. The expression of miR-223 was significantly higher in GC cells compared with normal epithelial cells (P<0.01).
Discussion
Based on a comprehensive assessment, this is the first report evaluating the diagnostic efficacy of circulating miR-223 in cancers. In this meta-analysis, we observed that circulating miR-223 had relatively higher diagnostic accuracy and yielded a combined AUC of 0.89 with 81% pooled sensitivity and 84% pooled specificity in identifying patients with cancers. The DOR is an index measuring of the effectiveness of a diagnostic test. In our study, the DOR value is 22 (95% CI: 16-31). Moreover, in our independent validation test, we observed similar diagnostic efficiency of plasma miR-223 whose expression level was greatly increased in both GC plasma and tissues. Additionally, compared with normal epithelial cells, the expression levels of miR-223 were remarkably higher in GC cells. All of these results are convincing and the miR-223 expression pattern shows no differences in plasma, tissue and cells.
It is important for meta-analyses to examine the potential sources of heterogeneity before pooling the results of primary studies into summary estimates [21]. Based on this, we explored the heterogeneity of the study for both threshold and non-threshold effect. Firstly, there is no heterogeneity caused by threshold effect in this meta-analysis. Next, we further identify the heterogeneity caused by non-threshold effect. After meta-regression analysis, we considered that normalization control, cancer type as well as ethnicity might be the possible sources of heterogeneity in the study. Further, these enrolled studies set different control groups which can be mainly organized in two different categories: U6 and miRNA. Some studies considered that miRNA lacks sequence homology and has lower variability between cancer and normal controls, while other studies suggested that U6 is statistically superior to the most commonly used reference genes in the quantification of serum miRNAs. These disagreements could possibly result in significant heterogeneity among these studies. Moreover, significant heterogeneity might also exist due to remarkable expression differences of miR-223 in different types of cancers.
Circulating miRNAs, also known as cell-free miRNAs, are promising biomarkers for predicting early cancers. Characterized by their non-invasive nature, miRNAs have a great potential in early cancer detection as they are structurally stable, easy to be detected, and will facilitate the measurement of both sensitivity and specificity [22]. To date, many studies have reported the possibility of miR-223 to be a valuable biomarker for cancer screening. The diagnostic accuracy values, however, have been inconsistent among these studies. As a result, there is no agreement on whether miR-223 should be selected as a reliable biomarker for cancer screening. Hence, we performed this systemic meta-analysis to evaluate the pooled value of miR-223 as a novel molecule for its diagnostic potentials. Although miR-223 has been reported to be nearly exclusively expressed in bone marrow [23], its overexpression has also been observed in different types of cancers, such as esophageal carcinoma (EC) [24], hepatocellular carcinoma (HCC) [25], and GC [26]. In patients with early-stage cancers, the remarkably high levels of miR-223 in plasma could be explained by the following reason: In cancer microenvironment, many tumor-associated cells, such as dendritic cells, macrophages, myeloid cells and T cells, could release exosomes, which were responsible for shuttling both mRNAs and miRNAs to other cells or circulation. In some tumor-associated cells, miR-223 might be up-regulated and then delivered by exosomes into peripheral blood circulation [27]. For example, recent studies illustrate that the miR-223, released by macrophages, was shuttled into breast cancer cells in which it regulates the proliferation and invasiveness of these cancer cells [28]. Further, the endogenous plasma miRNAs exist in a form that is resistant to plasma RNase, indicating that miRNAs in plasma remain quite stable and is easy to detect [29]. Besides, differed from endoscopy, circulating miRNAs have striking advantages for their non-invasive feature and simplified procedures [30]. Also, when compared with classic biomarkers such as CEA or CA199, miRNAs seem to obtain significantly higher sensitivity and specificity [31].
This meta-analysis has several limitations that need to be acknowledged. Firstly, for the reason that the clinical values of miR-223 have been explored in cancer diagnosis only for recent years, a small sample size is involved in our meta-analysis. As a result, small-study effects are inescapable and it is necessary to strengthen our conclusion by further validations of miR-223 in a larger cohort study and in more independent studies. Secondly, standardized protocol, such as normalization control, which should be preferably followed across all studies, needs to be established in order to minimize protocol-based bias. Thirdly, as most included studies in this meta-analysis merely made a distinguishing between cancer patients and healthy controls, it is vital to identify or develop panels of miRNAs that can distinguish cancers from other diseases, especially those with similar symptoms of cancers. Last but not least, as shown in Table 1, most of included studies were from Asia and little on western populations. Therefore, more studies should be conducted on western populations.
In conclusion, our meta-analysis comprehensively suggested that circulating miR-223 could distinguish cancer patients from normal controls and the further validation in an independent cohort also indicated that plasma miR-223 functions as a promising non-invasive screening tool for early detection of GC. Further studies are warrant to validate these results.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81270476 and 81470830), the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801) and Jiangsu postgraduate scientific research and innovation projects (CXZZ13_0574).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, Moore M, Rydall A, Rodin G, Tannock I, Donner A, Lo C. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383:1721–30. doi: 10.1016/S0140-6736(13)62416-2. [DOI] [PubMed] [Google Scholar]
- 2.Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutictarget for personalized cancer treatment. Drug Resist Updat. 2014;17:89–95. doi: 10.1016/j.drup.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Alečković M, Kang Y. Regulation of cancer metastasis by cell-free miRNAs. Biochim Biophys Acta. 2014;1855:24–42. doi: 10.1016/j.bbcan.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, Robertson DJ, Boland CR, Giardello FM, Lieberman DA, Levin TR, Rex DK US Multi-Society Task Force on Colorectal Cancer. Optimizing adequacy of bowel cleansing for colonoscopy: re commendations from the US multi-society task forceon colorectal cancer. Gastroenterology. 2014;147:903–24. doi: 10.1053/j.gastro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Grande AJ, Silva V, Riera R, Medeiros A, Vitoriano SG, Peccin MS, Maddocks M. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev. 2014;11:CD010804. doi: 10.1002/14651858.CD010804.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Li Y, Zhang X, Jing J, Zhao X, Wang Y, Han C. Evaluating the significance of expression of CEA mRNA and levels of CEA and its related proteins in colorectal cancer patients. J Surg Oncol. 2014;109:440–4. doi: 10.1002/jso.23503. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Heo I, Kim VN. Modifications of small RNAs and their associated proteins. Cell. 2010;143:703–9. doi: 10.1016/j.cell.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida-Aoki N, Ochiya T. Interactions between cancer cells and normal cells via miRNAs in extracellular vesicles. Cell Mol Life Sci. 2015;72:1849–61. doi: 10.1007/s00018-014-1811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Wang C, Chen X, Yang C, Li K, Wang J, Dai J, Hu Z, Zhou X, Chen L, Zhang Y, Li Y, Qiu H, Xing J, Liang Z, Ren B, Yang C, Zen K, Zhang CY. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56:1871–9. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 11.Geng Q, Fan T, Zhang B, Wang W, Xu Y, Hu H. Five microRNAs in plasma as novel biomarkers for screening of early-stage non-small cell lung cancer. Respir Res. 2014;15:149. doi: 10.1186/s12931-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia W, Wu Y, Zhang Q, Gao G, Zhang C, Xiang Y. Identification of four serum microRNAs from a genome-wide serum microRNA expression profile as potentialnon-invasive biomarkers for endometrioid endometrial cancer. Oncol Lett. 2013;6:261–267. doi: 10.3892/ol.2013.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maclellan SA, Lawson J, Baik J, Guillaud M, Poh CF, Garnis C. Differential expression of miRNAs in the serum of patients with high-risk oral lesions. Cancer Med. 2012;1:268–74. doi: 10.1002/cam4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Wang L, Wu Z, Sun R, Jin H, Ma J, Liu L, Ling R, Yi J, Wang L, Bian J, Chen J, Li N, Yuan S, Yun J. Three dys regulated microRNAs in serum as novel biomarkers for gastric cancer screening. Med Oncol. 2014;31:298. doi: 10.1007/s12032-014-0298-8. [DOI] [PubMed] [Google Scholar]
- 15.Zheng G, Du L, Yang X, Zhang X, Wang L, Yang Y, Li J, Wang C. Serum microRNA panel as biomarkers for early diagnosis of colorectal adenocarcinoma. Br J Cancer. 2014;111:1985–92. doi: 10.1038/bjc.2014.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo X, Mao XH, Zou QM, Yu PW, Zuo QF, Li N, Tang B, Liu KY, Xiao B. Plasma microRNAs, miR-223, miR-21 and miR-218, asnovel potential biomarkers for gastric cancer detection. PLoS One. 2012;7:e41629. doi: 10.1371/journal.pone.0041629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu YD, Wang L, Sun C, Fan L, Zhu DX, Fang C, Wang YH, Zou ZJ, Zhang SJ, Li JY, Xu W. Distinctive microRNA signature is associated with the diagnosis and prognosis of acute leukemia. Med Oncol. 2012;29:2323–31. doi: 10.1007/s12032-011-0140-5. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, Lin D. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–42. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Wang C, Guan X, Liu Y, Li D, Zhou X, Zhang Y, Chen X, Wang J, Zen K, Zhang CY, Zhang C. Diagnostic and prognostic implications of a serum miRNA panel inoesophageal squamous cell carcinoma. PLoS One. 2014;9:e92292. doi: 10.1371/journal.pone.0092292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Jeon TY, Choi CI, Kim DH, Kim DH, Kim GH, Ryu DY, Lee BE, Kim HH. Validation of circulating miRNA biomarkers for predicting lymph node metastasisin gastric cancer. J Mol Diagn. 2013;15:661–9. doi: 10.1016/j.jmoldx.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity insystematic reviews of diagnostic tests. Stat Med. 2002;21:1525–37. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 22.Witwer KW. Circulating MicroRNA Biomarker Studies: Pitfalls and Potential Solutions. Clin Chem. 2015;61:56–63. doi: 10.1373/clinchem.2014.221341. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Kang S, Min KH, Woo Hwang K, Min H. Age-associated changes in microRNAexpression in bone marrow derived dendritic cells. Immunol Invest. 2013;42:179–90. doi: 10.3109/08820139.2012.717328. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Ajani JA, Gu J, Chang DW, Tan W, Hildebrandt MA, Huang M, Wang KK, Hawk E. MicroRNA expression signatures during malignant progression from Barrett’sesophagus to esophageal adenocarcinoma. Cancer Prev Res (Phila) 2013;6:196–205. doi: 10.1158/1940-6207.CAPR-12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M, Zhu Y, Zhao Q, Dong YW, Shao K, Wu A, Wu XZ. MicroRNA-223 regulates FOXO1 expression and cell proliferation. FEBS Lett. 2012;586:1038–43. doi: 10.1016/j.febslet.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 26.Eto K, Iwatsuki M, Watanabe M, Ishimoto T, Ida S, Imamura Y, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Baba H. The sensitivity of gastric cancerto trastuzumab is regulated by the miR-223/FBXW7 pathway. Int J Cancer. 2015;136:1537–45. doi: 10.1002/ijc.29168. [DOI] [PubMed] [Google Scholar]
- 27.Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB. Macrophagemicrovesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–95. doi: 10.1182/blood-2011-08-374793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodahl AR, Zeuthen P, Binder H, Knoop AS, Ditzel HJ. Alterations in circulating miRNA levels following early-stage estrogen receptor-positive breast cancer resection in post-menopausal women. PLoS One. 2014;9:e101950. doi: 10.1371/journal.pone.0101950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19:1568–75. doi: 10.3390/molecules19021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos J, Fernandes E, Ferreira JA, Lima L, Tavares A, Peixoto A, Parreira B, Correia da Costa JM, Brindley PJ, Lopes C, Santos LL. P53 and cancer-associated sialylated glycans are surrogate markers of cancerization of the bladder associated with Schistosoma haematobium infection. PLoS Negl Trop Dis. 2014;8:e3329. doi: 10.1371/journal.pntd.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Li S, Wei L, Liang X, Zhang H, Liu J. The correlation betweenpre-operative serum tumor markers and lymph node metastasis in gastric cancerpatients undergoing curative treatment. Biomarkers. 2013;18:632–7. doi: 10.3109/1354750X.2013.840800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


