Abstract
Background
The surplus of glycerol has increased remarkably as a main byproduct during the biofuel’s production. Exploiting an alternative route for glycerol utilization is significantly important for sustainability of biofuels.
Results
A novel biocatalyst that could be prepared from glycerol for producing 2-oxo-carboxylates was developed. First, Pseudomonas putida KT2440 was reconstructed by deleting lldR to develop a mutant expressing the NAD-independent lactate dehydrogenases (iLDHs) constitutively. Then, the Vitreoscilla hemoglobin (VHb) was heterologously expressed to further improve the biotransformation activity. The reconstructed strain, P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, exhibited high activities of iLDHs when cultured with glycerol as the carbon source. This cost-effective biocatalyst could efficiently produce pyruvate and 2-oxobutyrate from dl-lactate and dl-2-hydroxybutyrate with high molar conversion rates of 91.9 and 99.8 %, respectively.
Conclusions
The process would not only be a promising alternative for the production of 2-oxo-carboxylates, but also be an example for preparation of efficient biocatalysts for the value-added utilization of glycerol.
Keywords: Glycerol, Pseudomonas putida KT2440, Biocatalyst, NAD-independent lactate dehydrogenase, 2-Oxo-carboxylate
Background
The increasing demand for renewable fuel has resulted in the increasing production of biofuels. As an inevitable byproduct, glycerol is generated in both bioethanol and biodiesel production processes [1]. For instance, a large amount of glycerol is produced during fermentative production of bioethanol [1]. About 10 kg of crude glycerol will be generated from every 100 kg of biodiesel production by the transesterification of fats and oils with alcohol [2]. Significant amounts of glycerol surplus, created by the increasing bioethanol and biodiesels production, have given rise to a sharp drop in glycerol price [1–3]. The painfully low glycerol price has negatively impacted the development of biofuels industry [2]. Therefore, exploiting new value-added alternate ways of glycerol utilization is positive to improve the viability of the biofuel economy.
Glycerol can be converted into various high-value products via either chemical transformations or biological conversions [4, 5]. For example, high-value fine chemicals such as dihydroxyacetone, tartronic acid, and mesoxalic acid can be obtained by selective catalytic oxidation of glycerol or glycerol derivatives [6]. Due to its advantages of higher specificity, milder reaction conditions, and lower levels of chemical contaminants, biological conversion is more desirable for environment-friendly production of those valuable chemicals from glycerol [3, 4, 7].
A wide variety of microorganisms, including the Pseudomonas putida KT2440, are able to utilize glycerol as the carbon and energy source [8, 9]. Recent studies related to biotechnological production of fuels and chemicals from glycerol have focused on metabolites derived from glycerol-assimilation process such as 1,3-propanediol, succinic acid, 2,3-butanediol, and ethanol [10–13]. There are also some studies concerning the use of glycerol for cultures of the oil-accumulating microbes such as microalgae, bacteria, yeasts and other fungi. The lipids recovered from whole cells of these oil-accumulating microbes can be transesterified into biodiesel and possess a composition similar to that of plant-based oils [14–16].
Besides the source of lipids, whole cells of microbes with different enzymes could also be used in the biocatalysis processes [17]. For example, there is a large amount of potential enzymes in genome of P. putida KT2440, which evolutionarily endow this strain with capacities to host a variety of biodegradation pathways and enable it to withstand exposure to diverse types of aggressive aromatic compounds, such as naphthalene, 4-chloronitrobenzene, 2,4-xylenol and phenol [18]. P. putida KT2440 is a microorganism officially classified as Generally Recognized as Safe. Thus, the whole cells of the strain can be used as a robust biocatalyst for biotransformation in various applications. However, these potential enzymes, such as NAD-independent lactate dehydrogenases (iLDHs, which involved in the catabolism of lactate) [19] and the enzymes involved in the catabolism of catechol (encoded by a cat cluster) [20] are generally strictly regulated, requiring expensive or toxic inducers for their expressions.
Since the release of complete sequence of P. putida KT2440’s genome, metabolic networks of the P. putida KT2440 have been reconstructed and the regulation mechanisms of various potential enzymes have been disclosed. Here, we reported a case study of production of 2-oxo-carboxylates, using glycerol as the carbon source for the biocatalyst preparation, which based on the regulation mechanism of lactate utilization [19]. The transcriptional repressor LldR of lactate utilization operon was deleted in the P. putida KT2440. Whole cells of the recombinant strain were confirmed to constitutively exhibit the activities of iLDHs catalyzing the oxidation of 2-hydroxy-carboxylates. Then, two important 2-oxo-carboxylates: pyruvate and 2-oxobutyrate (2-OBA) were produced with whole cells of the recombinant strain prepared from glycerol as the catalyst. The process provides a promising alternative for the value-added utilization of biotechnologically produced glycerol.
Results and discussion
Regulatory networks of glycerol and lactate metabolism in P. putida KT2440
Owing to its versatile metabolic activities, P. putida KT2440 can use various organics as carbon and energy sources, which make this strain an ideal industrial microorganism used for biotransformation and biodegradation [21]. However, most of the metabolism networks are subject to the strict regulation. Take glycerol and lactate as examples, both of these two compounds can be used as carbon and energy sources for P. putida KT2440 [9, 22]. During the glycerol utilization process, the specific enzymes related to the glycerol metabolism, including GlpF (a glycerol transporter encoded by glpF), GlpK (a glycerol kinase encoded by glpK), and GlpD (a glycerol-3-phosphate dehydrogenase encoded by glpD), will be generally induced [9]. The expressions of these genes are regulated by GlpR, a DeoR family transcriptional regulator, which is encoded by glpR (PP1074) [9, 23, 24]. The expression of glpR is not affected by glycerol [9]. The primary structure and N-terminal helix-turn-helix (HTH) DNA-binding motif of GlpR in Escherichia coli K12 have already been identified [25]. Moreover, previous research has shown that the GlpR of E. coli K12 is a tetramer under native conditions [26]. Since the GlpR from E. coli K12 and P. putida KT2440 share the high consensus positions (71.0 %) and identity positions (54.8 %), we conjectured that the GlpR from P. putida KT2440 might be a tetramer and possess N-terminal HTH DNA-binding motifs as well. The structure of C-terminal effector-binding domain of DeoR from Bacillus subtilis has been determined [27]. In view of the fact that the GlpR belongs to the DeoR family transcriptional regulator [25], the GlpR might also possess the similar C-terminal effector-binding domains. Based on these backgrounds mentioned above, the hypothetic schematics of the regulatory networks of the GlpR-depended glycerol metabolism in P. putida KT2440 are shown in Fig. 1. As shown in Fig. 1a, when P. putida KT2440 is cultured with the glycerol as a carbon source, the glycerol from the extracellular environment will be transported into cytoplasm (mediated by GlpF) [24]. The sn-glycerol-3-P (G3P) produced by the substrate phosphorylation of glycerol (mediated by GlpK, the glycerol kinase) will be generated via the effector-independent expression of glp genes, which occurs in a low-probability stochastic way [24]. And then the repression of glp genes mediated by GlpR will be derepressed by the increasing intracellular G3P [24]. As an effector for GlpR, G3P can bind to the effector-binding domains of GlpR, and then will prevent the HTH DNA-binding domains of GlpR from binding to the DNA operator sequence of glp regulon [24, 25, 27]. On the contrary, the free tetrameric assembly of GlpR will tightly bind to the DNA operator sequence and inhibit the expressions of glp genes when the strain cultured without glycerol (Fig. 1b) [24, 27].
Fig. 1.
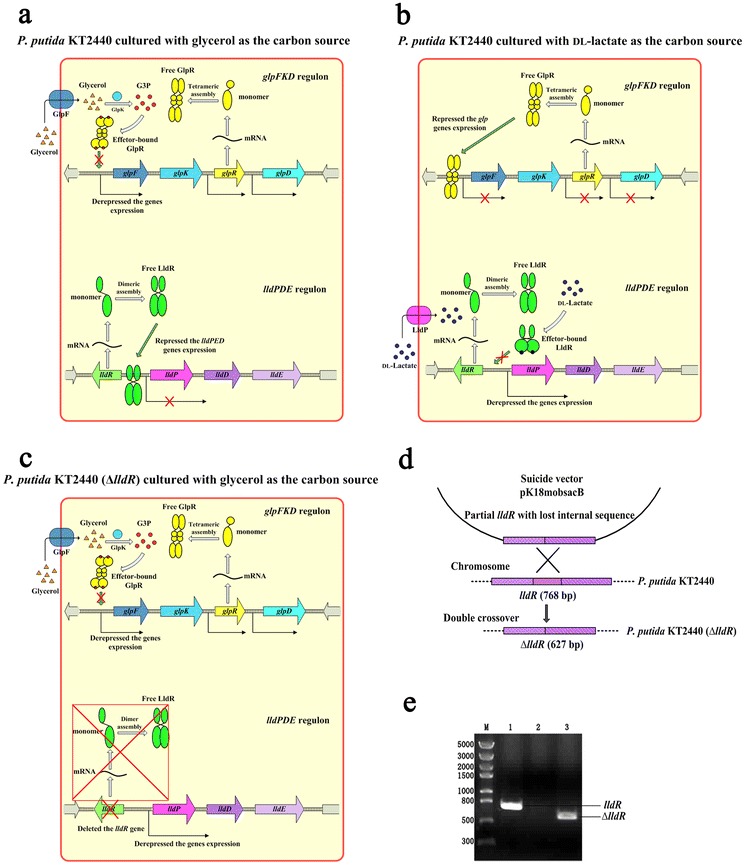
The hypothetic schematics of regulatory networks of glycerol and lactate metabolism in P. putida KT2440, and the construction of P. putida KT2440 (ΔlldR). a The derepression of the glp genes expression is occurring when the strain cultured in MSM with glycerol as carbon source. On the other hand, the expressions of lldPDE genes are repressed by the LldR, without the lactate as inducer. b The derepression of the lldPDE genes expression is occurring when the strain cultured in MSM with dl-lactate as carbon source. On the contrary, the free tetrameric assembly of GlpR will tightly bind to the DNA operator sequence with the DNA-binding domains, and inhibit the expressions of glp genes. c If the lldR gene is deleted, the repression of the lldPDE genes expression will be damaged, owing to the absence of the functional LldR. And the lldPDE genes will still fully express, even without lactate as the inducer. d Diagram illustrating the disruption of the lldR mediated by homologous double crossover. e Analysis of PCR fragments to confirm lldR disruption. Lane M molecular mass standard (λDNA/HindIII); lane 1 product amplified with P. putida KT2440 genomic DNA as the template; lane 2 product amplified with water as the template (negative control); lane 3 product amplified with P. putida KT2440 (ΔlldR) genomic DNA as the template. The PCRs were performed with primers lldRk.f and lldRk.r
The similar regulation network also exists in the lactate metabolism of P. putida KT2440. The lldPDE operon, which is responsible for lactate utilization in most Pseudomonas strains including P. putida KT2440, has been studied in previous reports [19, 28–31]. The lldPDE operon comprises 3 genes, lldP (encoding a lactate permease), lldD (encoding an l-iLDH), and lldE (encoding a d-iLDH) [19, 22]. The l-iLDH and d-iLDH are mainly responsible for the oxidation of l-lactate and d-lactate to pyruvate, respectively [19, 28, 31]. However, the expressions of lldD and lldE are both repressed by the regulator LldR which is encoded by upstream adjacent gene lldR [19]. The previous study reported that the LldR from Corynebacterium glutamicum is a homodimer assembled by domain swapping [32]. The N-domain of this regulator contains a typical winged HTH (WHTH) DNA-binding domain [32, 33]. And the C-terminal domain is assumed to play the ligand-binding role [32]. As the primary structure predicted by NCBI, the monomer of LldR from P. putida KT2440 comprises an N-terminal WHTH DNA-binding and a C-terminal ligand-binding domain, which is coincident with the typical region features of many members of the GntR family [34]. The hypothetic schematics of the regulatory networks of the LldR-mediated lactate metabolism in P. putida KT2440 have also been shown in Fig. 1. As shown in Fig. 1a, while the strain cultured in the medium without lactate, the free LldR homodimer can bind to the promoter region of the lldPDE operon with the WHTH DNA-binding domains, and will inhibit the expressions of lldPDE genes downstream. However, the derepression of LldR to the lldPDE genes is occurring when lactate exists in the growing environment of the strain. As an effector of LldR, the lactate can bind to the C-terminal ligand-binding domains. Then, the effector-bound LldR will lose the ability of binding to the DNA promoter sequence of lldPDE regulon (Fig. 1b).
Reconstruction of P. putida KT2440 lldR deletion mutant
As mentioned before, the presence of lactate is necessary for the expressions of l-iLDH and d-iLDH [19, 31, 35]. As a result, the hyperosmotic medium caused by high concentration of lactate becomes a major limitation for high-density culture, and the indispensable lactate addition raises the cost of biocatalysts preparation. Considering the versatile applications of P. putida KT2440 in biocatalysis, it is rather desirable to prepare efficient biocatalyst from a more cost-effective substrate, such as glycerol, with the required enzymes iLDHs. To achieve this goal, the regulatory network of lactate utilization was reconstructed. After deleting the lldR gene, it seems likely that the regulatory network would be broken down because of the absence of the functional LldR. Therefore, when cells incubated with the glycerol as the sole carbon source, the lldPDE genes would still fully express, even without the induction of lactate (Fig. 1c). To explore this possibility, we disrupted the lldR gene which encodes negative regulator LldR in P. putida KT2440. The suicide plasmid pK18mobsacB which mediated the homologous recombination was used for deleting the lldR gene (Fig. 1d) [36]. The disruption of the gene lldR was verified by PCR (Fig. 1e). The result strain is named P. putida KT2440 (ΔlldR).
Effect of inactivation of lldR on iLDHs expression
The wild-type P. putida KT2440 and P. putida KT2440 (ΔlldR) were cultured in 500-mL baffled shake flasks each containing 100 mL minimal salt medium (MSM) [31] supplied with 5 g/L dl-lactate or glycerol as the carbon source. And 1 mM octanoate, as the co-feeder, was added to the MSM with glycerol to shorten lag phase [23]. To investigate the effect of inactivation of lldR on iLDHs expression, the activities of l-iLDH and d-iLDH in crude cell extracts of P. putida KT2440 and P. putida KT2440 (ΔlldR) were assayed, with 2,6-dichloroindophenol (DCIP) as the artificial electron acceptor and 20 mM l- or d-lactate as the electron donor. As shown in Table 1, when the P. putida KT2440 was cultured in the medium with dl-lactate as the sole carbon source, the enzymes activities of l-iLDH and d-iLDH were 161.4 nmol/min mg protein and 332.9 nmol/min mg protein, respectively. However, neither l-iLDH nor d-iLDH activity was detectable in P. putida KT2440 when cultured with glycerol. Comparatively, when the P. putida KT2440 (ΔlldR) was incubated in the MSM with dl-lactate as the carbon source, the activity of l-iLDH was 220.9 nmol/min mg protein and d-iLDH was 471.5 nmol/min mg protein. While incubated in the MSM containing the glycerol as the carbon source, this ΔlldR mutant also exhibited high activities of iLDHs, 348.2 nmol/min mg protein and 771.0 nmol/min mg protein for l-iLDH and d-iLDH, respectively. These results revealed that the repression effects of LldR on l-iLDH and d-iLDH expressions were removed by the disruption of lldR gene. When cultured with glycerol, the iLDHs activities of the P. putida KT2440 (ΔlldR) were not impaired, compared with that cultured with dl-lactate. Therefore, the P. putida KT2440 (ΔlldR), in which l-iLDH and d-iLDH are expressed constitutively, has the potential to efficiently produce pyruvate from lactate with glycerol as the cost-effective culture substrate.
Table 1.
Activities of iLDHs in crude cell extracts of P. putida KT2440 and P. putida. KT2440 (∆lldR) cultured with different growth substrates
| Growth substrate | Strain | Enzyme activity (nmol/min mg protein)a | |
|---|---|---|---|
| l-iLDH | d-iLDH | ||
| dl-Lactate | P. putida KT2440 | 161.4 ± 4.6 | 332.9 ± 4.3 |
| P. putida KT2440 (∆lldR) | 220.9 ± 7.0 | 471.5 ± 3.5 | |
| Glycerol | P. putida KT2440 | ND | ND |
| P. putida KT2440 (∆lldR) | 348.2 ± 11.2 | 771.0 ± 9.0 | |
ND not detected
aActivities of d-iLDH and l-iLDH were examined with 20 mM d-lactate or 20 mM l-lactate. DCIP was used as the electron acceptor. Results are mean ± SD of three parallel replicates
Vitreoscilla hemoglobin (VHb) enhances the lactate oxidation
It has been revealed in a previous study that iLDHs from P. stutzeri SDM could not oxidize lactate with oxygen as the directly electron acceptor [37]. The lldPDE operon organization is similar in P. putida KT2440 and P. stutzeri SDM, and the lactate utilization genes between these two strains show strikingly high homology [19, 29, 38]. It is inferred that the electron produced in the lactate oxidation process might terminally transfer to the oxygen, a final electron acceptor, through the electron transport chain in P. putida KT2440, as well as in P. stutzeri SDM [37].
Vitreoscilla hemoglobin (VHb) is a soluble homodimeric globin encoded by vgb, a 438 bp gene discovered in Vitreoscilla sp. [39]. It is the first bacterial hemoglobin whose structure and function have been well characterized [39, 40]. Since the gene (vgb) encoding VHb has been cloned [41, 42], its heterologous expression has become an engineering strategy widely used to increase production of a diverse of bioproducts and facilitate the bioremediation [43]. In this study, the vgb gene was amplified with the primers vgb.f and vgb.r from the vector pET28b-RgDAAO-VHb. Then, the 438 bp vgb fragment was ligated to HindIII and BamHI double-digested pBSPPcGm, a broad-host-range constitutive vector containing a Pc promoter [44], to produce pBSPPcGm-vgb (Fig. 2a). As shown in Fig. 2b, the vgb gene has been successfully cloned and inserted into the pBSPPcGm with the corresponding restriction enzyme sites, obtaining the pBSPPcGm-vgb. The vector was transferred into P. putida KT2440 and P. putida KT2440 (ΔlldR) by electroporation to produce P. putida KT2440/pBSPPcGm-vgb and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, respectively.
Fig. 2.
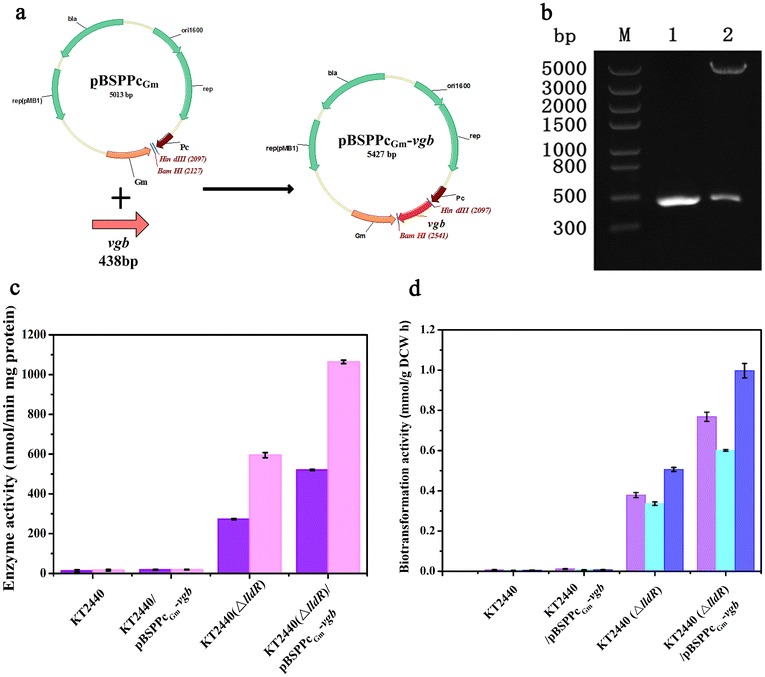
Construction of the vgb expressing vector and comparisons of iLDHs activities and biotransformation rates toward pyruvate production between different P. putida KT2440 strains. a Construction of recombinant vector pBSPPcGm-vgb. vgb, the gene encoding VHb. pBSPPcGm, a broad-host-range constitutive vector containing a P c promoter. The vgb gene was inserted into the pBSPPcGm-vgb in the corresponding sites, to generate the plasmid pBSPPcGm-vgb. b Verification of pBSPPcGm-vgb. Lane M molecular mass standard (λDNA/HindIII); lane 1 product amplified with pET28b-RgDAAO-VHb as the template; lane 2 double enzymes digestion (HindIII and BamHI) of recombinant vector pBSPPcGm-vgb. c Activities of l-iLDH (violet bars) and d-iLDH (light magenta bars) in crude cell extracts of P. putida KT2440 and its derivatives were examined with DCIP as the artificial electron acceptor and 20 mM l- or d-lactate as the electron donor. Results are mean ± SD of three parallel replicates. d The biotransformation rates of pyruvate production by whole cells of different P. putida KT2440 strains. The biotransformations were conducted with the whole cells of P. putida KT2440, P. putida KT2440/pBSPPcGm-vgb, P. putida KT2440 (ΔlldR) and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb with 100 mM l-lactate (violet bars), 100 mM d-lactate (light cyan bars) and 100 mM dl-lactate (blue bars) as the substrates. The concentrations of the pyruvate were measured by HPLC. Results are mean ± SD of three parallel replicates
To explore the effect of introduction of VHb in lactate oxidation, the l-iLDH and d-iLDH activities in the crude cell extracts of P. putida KT2440, P. putida KT2440 (ΔlldR), P. putida KT2440/pBSPPcGm-vgb and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, cultured with glycerol, were assayed. As the results shown in Fig. 2c, the introduction of VHb did not increase the activities of iLDHs in P. putida KT2440 cultured with the glycerol as carbon source. The enzymes activities of l-iLDH and d-iLDH in P. putida KT2440 (ΔlldR) were 273.0 nmol/min mg protein and 595.0 nmol/min mg protein, respectively. However, compared with P. putida KT2440 (ΔlldR), both the activities of l-iLDH and d-iLDH in P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb significantly increased to 520.7 nmol/min mg protein and 1063.9 nmol/min mg protein, respectively, almost twofold higher than those in P. putida KT2440 (ΔlldR).
Effect of VHb introduction in whole-cell biocatalysis
It was also investigated if the introduction of VHb could affect the whole cells biocatalysis activities. The biocatalysis reactions were conducted at 30 °C in phosphate buffer (pH 7.4) for 6 h, with 10.5 g dry cell weight (DCW)/L of P. putida KT2440, P. putida KT2440/pBSPPcGm-vgb, P. putida KT2440 (ΔlldR) and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, which cultured with glycerol, as the biocatalysts, respectively. l-Lactate, d-lactate, and racemic lactate at 100 mM were used as the substrates. The biocatalysis reactions were carried out in the presence of 30 mM ethylenediaminetetraacetic acid (EDTA), which could remove bivalent ions necessary for 2-keto-acid decarboxylase-catalyzed reactions [45, 46], and then could block the degradation of 2-oxo-carboxylates. As shown in Fig. 2d, via 6 h biotransformation, no pyruvate production was detected when either P. putida KT2440 or P. putida KT2440/pBSPPcGm-vgb was used, which was in correspondence to the result of the activities assays of iLDHs of these two strains. However, both P. putida KT2440 (ΔlldR) and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb have the ability to oxidize the two enantiomers of lactate. For P. putida KT2440 (ΔlldR), the oxidation rates toward l-lactate, d-lactate and dl-lactate to pyruvate were 0.38 mmol/g DCW h, 0.34 mmol/g DCW h and 0.51 mmol/g DCW h, respectively. And for P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, the oxidation rates toward these three kinds of lactate have remarkably increased to 0.77 mmol/g DCW h, 0.60 mmol/g DCW h and 1.00 mmol/g DCW h, respectively (Fig. 2d). As expected, the reconstructed strain with heterologously expressed VHb exhibited about twofold higher biotransformation activities than the strain without VHb expression. These results revealed that the introduction of VHb into the recombinant P. putida KT2440 (ΔlldR) indeed significantly enhanced the whole cells biocatalysis activities of lactate oxidation to produce pyruvate. Furthermore, the pyruvate production rates from dl-lactate were significantly higher than which from either l-lactate or d-lactate alone (Fig. 2d). l-iLDH and d-iLDH catalyze the oxidation of l-lactate and d-lactate, respectively. The higher biotransformation activity toward dl-lactate might be due to the fact that both isomers in dl-lactate would be simultaneously oxidized by l-iLDH and d-iLDH in these recombinant strains. Compared with optical pure lactate, the low price and large sources of racemic lactate make it become a more cost-effective substrate to produce pyruvate. Based on the results above, the P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, a recombinant strain with constitutive iLDHs and heterologously expressed VHb, has the potential to efficiently produce 2-oxo-carboxylates from 2-hydroxy-carboxylates.
Pyruvate and 2-OBA production through whole-cell biocatalysis
The oxidation of two most important members of 2-hydroxy-carboxylates, lactate and 2-hydroxybutyrate (2-HBA) that are catalyzed by iLDHs, have been studied in previous studies [37, 47]. In this study, the biocatalytic oxidation of racemic lactate (100 mM) and 2-HBA (100 mM) were carried out, with 10.5 g DCW/L of whole cells of P. putida KT2440, P. putida KT2440/pBSPPcGm-vgb, P. putida KT2440 (ΔlldR) and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb, which were prepared from glycerol, as the biocatalysts, respectively. The biocatalysis reactions were conducted at 30 °C in phosphate buffer (pH 7.4) with the presence of 30 mM EDTA. As shown in Tables 2 and 3, the racemic lactate (100 mM) and 2-HBA (100 mM) were completely oxidized into pyruvate and 2-OBA, via 6-h bioconversion with P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb as the biocatalyst. The yields of pyruvate and 2-OBA with P. putida KT2440 (ΔlldR) were 50.9 and 74.7 %, respectively. However, the yields of these two productions with P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb were 91.9 and 99.8 %, respectively, about 1.8-fold and 1.3-fold higher than which catalyzed by P. putida KT2440 (ΔlldR). The final concentrations of pyruvate and 2-OBA produced by P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb were 90.9 mM and 99.3 mM, respectively.
Table 2.
Comparison of pyruvate productions by whole cells of P. putida KT2440, P. putida/pBSPPcGm-vgb, P. putida KT2440 (ΔlldR), and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb
| Strain | Pyruvate (mM) | Yield (%)a | Productivity (mmol/g DCW h) |
|---|---|---|---|
| KT2440 | 0 | 0 | 0 |
| KT2440/pBSPPcGm-vgb | 0 | 0 | 0 |
| KT2440 (ΔlldR) | 50.60 ± 0.38 | 50.9 | 0.81 ± 0.005 |
| KT2440 (ΔlldR)/pBSPPcGm-vgb | 90.85 ± 0.75 | 91.9 | 1.43 ± 0.002 |
The initial dl-lactate concentration was 100 mM. The biocatalysis reactions were conducted at 30 °C in phosphate buffer (pH 7.4) for 6 h with 10.5 g DCW/L of biocatalysts prepared from glycerol. Results are mean ± SD of three parallel replicates
aThe yields of pyruvate were calculated based on the actual initial concentrations of dl-lactate measured by HPLC
Table 3.
Comparison of 2-OBA productions by whole cells of P. putida KT2440, P. putida KT2440/pBSPPcGm-vgb, P. putida KT2440 (ΔlldR), and P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb
| Strain | 2-OBA (mM) | Yield (%)a | Productivity (mmol/g DCW h) |
|---|---|---|---|
| KT2440 | 0 | 0 | 0 |
| KT2440/pBSPPcGm-vgb | 0 | 0 | 0 |
| KT2440 (ΔlldR) | 75.52 ± 1.59 | 74.7 | 1.20 ± 0.03 |
| KT2440 (ΔlldR)/pBSPPcGm-vgb | 99.31 ± 1.45 | 99.8 | 1.58 ± 0.02 |
The initial dl-2-HBA concentration was 100 mM. The biocatalysis reactions were conducted at 30 °C in phosphate buffer (pH 7.4) for 6 h with 10.5 g DCW/L of biocatalysts prepared from glycerol. Results are mean ± SD of three parallel replicates
aThe yields of 2-OBA were calculated based on the actual initial concentrations of dl-2-HBA measured by HPLC
Many researches have focused on the conversions of the inexpensive glycerol into high-value products, such as fine chemicals [6] and biodiesels [16], via the microbial fermentation. Furthermore, glycerol can also be used as a carbon source and energy source to support the growth of many industrial microorganisms [8]. The development of biotechnology of glycerol utilization processes will allow the biofuel industry to be more competitive.
Compared with lactate, glycerol is a more cost-effective green substrate suitable for preparation of the biocatalyst containing the iLDHs. In 2006 and 2007, the spot price of the heat-stable lactic acid, as posted in the Chemical Marketing Reporter, was about $0.70 per pound [48]. In contrast, the prices of refined glycerol and crude glycerol were approximately $0.30 and $0.050 per pound, respectively, obviously cheaper than the lactic acid [49]. Furthermore, as a byproduct of biofuels, large amounts of glycerol have become a waste stream. Making good use of this waste stream would not only lower the production costs, but also contribute to the sustainable development of the biofuels industry. After reconstructing the lactate utilization regulatory network by deleting the LldR regulator in P. putida KT2440, the ΔlldR mutant exhibited the outstanding constitutive iLDHs activities when cultured with glycerol as the carbon source (Fig. 3). On the other hand, a constitutive vector with high expression strengths and broad host ranges was used for the introduction of VHb, which can enhance the lactate oxidation activities and the yields of the 2-oxo-carboxylates significantly (Fig. 3). This beneficial effect of VHb heterologous expression may be the result of binding more oxygen and delivering to the respiratory chain [43]. It is also feasible to apply this VHb introduction technology in other aerobic biotransformations to increase production efficiency.
Fig. 3.
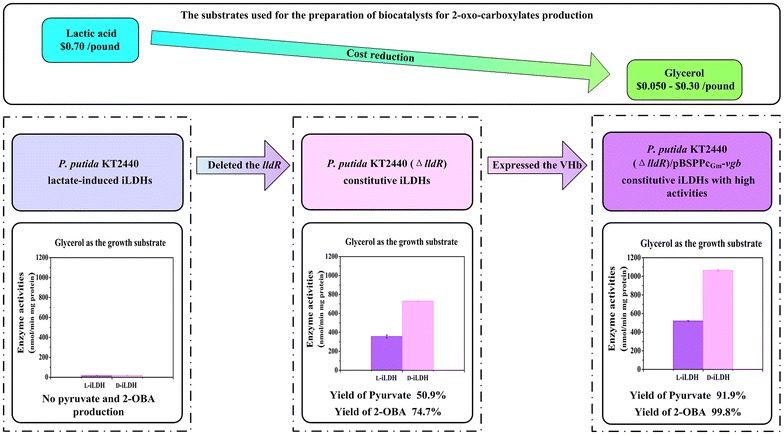
The optimization process of the biocatalysts prepared with glycerol
Pyruvate and 2-OBA are two important platform compounds which have been widely applied in the chemical, drug, and food industries [47, 50]. Pyruvate has been used as a weight-control dietary supplement, a supplemental nutrient, and an antioxidant which can protect the brain and other tissues from the oxidative stress [50–53]. 2-OBA is an important intermediate widely used in biosynthesis of l-isoleucine, d-2-hydroxybutyrate and 1-propanol [54–56]. Furthermore, 2-OBA could be bioconverted into a non-natural amino acid l-homoalanine, which is a key chiral precursor for production of levetiracetam, brivaracetam, and ethambutol [57, 58]. Owing to the mild reaction conditions, high substrate conversion efficiency and simple compositions of the reaction mixture which is contributed to the convenience of recovery, whole cell catalysis becomes the preferred method for pyruvate and 2-OBA production [47, 50]. For example, Pseudomonas stutzeri SDM, which contains inducible iLDHs, has been reported to have good ability to produce pyruvate and 2-OBA from lactate and 2-HBA as the substrates, respectively [37, 47]. Whole cells of P. stutzeri SDM with iLDHs must be prepared with lactate as the carbon source. Although the concentrations of pyruvate and 2-OBA reported here were lower than these previous reports, this work disclosed a novel biocatalyst which could be prepared with glycerol as a more cost-effective substrate.
Conclusions
Taking the 2-oxo-carboxylates production as an example, we developed a process of using biofuel’s byproduct glycerol for biocatalyst preparation. After deleting the LldR and heterologously expressing VHb in P. putida KT2440, the recombinant P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb with high iLDHs activities was cost-effectively prepared from glycerol. Using the whole cells as biocatalyst, 90.9 mM pyruvate and 99.3 mM 2-OBA were produced in 6 h from 100 mM dl-lactate and dl-2-HBA, respectively. The process demonstrated an option for effective utilization of the low-cost and renewable substrates through recombining the metabolic networks, based on the regulation mechanism, with the goal of producing the high-value chemicals.
Methods
Chemicals and biochemicals
Racemic lactate, l-lactate, octanoate, phenylmethanesulfonyl fluoride (PMSF), and DCIP were all purchased from Sigma. d-Lactate and racemic 2-HBA were obtained from Fluka and TCI, respectively. Restriction enzymes and T4 DNA ligase were purchased from Thermo Fisher Scientific Inc. FastPfu DNA polymerase was purchased from TransGen Biotech. All other chemicals were of reagent grade.
Bacterial strains and culture conditions
Bacterial strains, plasmids, and oligonucleotide primers used in this study are listed in Table 4. For DNA manipulations and for pre-cultures, E. coli and P. putida strains were grown in Luria–Bertani (LB) medium [59, 60] at 37 and 30 °C, respectively. Antibiotics at the following concentrations: kanamycin at 50 μg/mL, ampicillin at 100 μg/mL, and gentamicin at 30 μg/mL were added when necessary. For determination of iLDHs activities in crude cell extracts and preparation of whole-cell catalysts, P. putida KT2440 and its derivatives were inoculated into 500-mL baffled shake flasks each containing 100 mL MSM supplemented with 5 g/L dl-lactate or glycerol as the carbon source at 30 °C and 120 rpm for 12 h, respectively. And 1 mM octanoate was added to the glycerol MSM as the co-feeder to shorten lag phase [23].
Table 4.
Strains, plasmids, and oligonucleotide primers used in this study
| Name | Relevant characteristic | Reference |
|---|---|---|
| Strains | ||
| P. putida KT2440 | Wild-type; capable of dl-lactate utilizing | ATCC |
| P. putida KT2440 (ΔlldR) | lldR deletion strain of P. putida KT2440 | This study |
| P. putida KT2440/pBSPPcGm-vgb | P. putida KT2440 harboring pBSPPcGm-vgb; Gmr | This study |
| P. putida KT2440 (ΔlldR)/pBSPPcGm-vgb | P. putida KT2440 (ΔlldR) harboring pBSPPcGm-vgb; Gmr | This study |
| E. coli DH5α | λ− ϕ80lacZ∆M15 ∆ (lacZYA-argF) U169 recA1 endA1 hsdR17 (r−K, m+K) supE44 thi-1 gyrA relA1; used for gene clone | Invitrogen |
| E. coli BL21 (pET28b-RgDAAO-VHb) | E. coli BL21 harboring pET28b-RgDAAO-VHb; Kmr | Professor Sheng Yanga |
| Plasmids | ||
| pK18mobsacB | Allelic exchange vector, oriColE1 Mob+, lacZα, sacB; Kmr | [36] |
| pKLR | A fragment from KT2440 genome containing whole length of lldR was inserted in pK18mobsacB; Kmr | This study |
| pKSR | pKLR was completely digested by pstI, and then the large fragment was self-ligated; as a result, only partial length of lldR was inserted into pK18mobsacB; Kmr | This study |
| pBSPPcGm | A constitutive vector with high expression strengths; Gmr | [44] |
| pET28b-RgDAAO-VHb | pET28b containing Rg-daao gene and vgb gene,T7 promoter; Kmr | Professor Sheng Yanga |
| pBSPPcGm-vgb | pBSPPcGm containing gene vgb; Gmr | This study |
| Primers | Sequences (5′ → 3′) and properties | |
| lldRk.f | GAATTCATGGTTTTTGATCAGGTACGC (EcoRI) | This study |
| lldRk.r | AAGCTTTCAGCGCCCGCTGCGCCGCTCT (HindIII) | This study |
| vgb.f | CCCAAGCTTAGGAGACAGTAATGTTAGACCAGCAAACCATTA (HindIII) | This study |
| vgb.r | CGCGGATCCTTCAACCGCTTGAGCGTA (BamHI) | This study |
ATCC American Type Culture Collection
aForm Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Gene knockout procedure
Genomic DNA of P. putida KT2440 was extracted through the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). The lldR gene encoding the regulator LldR was amplified by PCR using lldRk.f and lldRk.r as the primers with the genomic DNA of P. putida KT2440 as the template, and then cloned into EcoRI and HindIII digested pK18mobsacB to form a new plasmid pKLR. Plasmid pKLR was completely digested by PstI, and the large fragment was self-ligated using T4 DNA ligase to form pKSR that contained the deleted version of lldR. After being transferred into P. putida KT2440 by electroporation, the first crossover cells containing the integration of the plasmid pKSR into the chromosome of P. putida KT2440 were selected on LB plate supplemented with 50 μg/mL kanamycin. The second crossover cells were singled out by culture on LB plates containing 10 % (w/v) sucrose [22]. The resulting deletion mutant was designated as P. putida KT2440 (ΔlldR). All the constructed strains were validated by PCR and DNA sequencing.
Cloning and expression of VHb in P. putida KT2440 and its derivatives
For the construction of VHb expression plasmid, VHb encoding gene (vgb) was amplified by PCR using a pair of primers, vgb.f and vgb.r, with plasmid pET28b-RgDAAO-VHb as a template. The PCR product was first ligated to the pEASY-Blunt vector, and the resulting plasmid was named pEASY-Blunt-vgb and sequenced. And then, after digested with HindIII and BamHI, the gel-purified vgb fragment was cloned into pBSPPcGm, a constitutive vector with high expression strengths [44], at the corresponding sites, to result the plasmid pBSPPcGm-vgb. Plasmid pBSPPcGm-vgb was transferred into P. putida KT2440 and its derivatives by electroporation. The P. putida KT2440 and its derivatives, which harboring the recombinant vector pBSPPcGm-vgb were selected on LB plate supplemented with 30 μg/mL gentamicin.
Biocatalyst preparation
Whole cells of P. putida strains cultured in MSM containing 5 g/L glycerol and 1 mM octanoate were harvested by centrifugation at a speed of 6000 rpm for 10 min. After washing twice with phosphate buffer (pH 7.4), whole cells were suspended in phosphate buffer and ready for the following study. Optical densities of whole cells were assayed and converted to DCW according to the previous report [22].
Enzymatic activity assays
Whole cells of the P. putida strains grown in MSM containing 5 g/L glycerol and 1 mM octanoate were disrupted by sonication (Sonics 500 W/20 kHz, USA) on ice bath. The disrupted cells were centrifuged for 20 min at 12,000 rpm, and the supernatants were used as crude cell extracts [37]. The activities of iLDHs were assayed by monitoring the change in absorbance at 600 nm corresponding to the reduction of DCIP at 30 °C with a UV/visible spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences, USA) [61]. The reaction was carried out in 0.8 mL of 50 mM Tris–HCl, pH 7.5, containing 20 mM l- or d-lactate, 0.0625 mM DCIP, and 50 μL of crude cell extracts [22].
Whole-cell biocatalysis for production of pyruvate and 2-OBA
The reactions were carried out at 30 °C and 120 rpm in phosphate buffer (pH 7.4) containing 10.5 g DCW/L of biocatalysts, 30 mM EDTA, and 100 mM racemic 2-hydroxy-carboxylates. The concentrations of pyruvate and 2-OBA in the reaction mixtures were quantitatively analyzed by high-performance liquid chromatography (HPLC) using an Aminex HPX-87H column (Bio-Rad) and the mobile phase (10 mM H2SO4) at 0.4 mL/min flow rate at 55 °C [37].
Authors’ contributions
YW carried out the lldR gene knockout and VHb cloning and expression, participated in the enzymatic activity assays and drafted the manuscript. YZ carried out the enzymatic activity assays and helped to revise the manuscript. TJ performed the data analysis and helped to draft the manuscript. JM executed the whole-cell biocatalysis experiments and helped to revise the manuscript. BS performed the HPLC analysis and helped to revise the manuscript. CG conceived of the study, and participated in its design and drafted the manuscript. CY participated in the interpretation of data for the work and helped to revise the manuscript. CM participated in the design of the study and coordination and revised the manuscript. PX conceived of the study and coordination, contributed reagents and materials, and helped to revise the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to sincerely and genuinely thank Prof. Sheng Yang (Key Laboratory of Synthetic Biology, Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for providing the vector pET28b-RgDAAO-VHb. The study was supported by National Natural Science Foundation of China (31270090, 31170052, 31270856), Chinese National Program for High Technology Research and Development (2014AA021206, 2011AA02A207), and Young Scholars Program of Shandong University (2015WLJH25).
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- iLDHs
NAD-independent lactate dehydrogenases
- 2-OBA
2-Oxobutyrate
- HTH
Helix-turn-helix
- WHTH
Winged HTH
- G3P
sn-glycerol-3-P
- VHb
Vitreoscilla hemoglobin
- MSM
Minimal salt medium
- DCW
Dry cell weight
- EDTA
Ethylenediaminetetraacetic acid
- 2-HBA
2-Hydroxybutyrate
- PMSF
Phenylmethanesulfonyl fluoride
- DCIP
2,6-Dichloroindophenol
- LB
Luria–Bertani
- HPLC
High-performance liquid chromatography
Contributor Information
Yujiao Wang, Email: wangeva-jiao@163.com.
Yingxin Zhang, Email: aicalie@163.com.
Tianyi Jiang, Email: jiangtianyi12@163.com.
Jingjing Meng, Email: 1518144482@qq.com.
Binbin Sheng, Email: binbinsdu@126.com.
Chunyu Yang, Email: ycy21th@sdu.edu.cn.
Chao Gao, Email: jieerbu@sdu.edu.cn.
Ping Xu, Email: pingxu@sjtu.edu.cn.
Cuiqing Ma, Phone: 86-531-88364003, Email: macq@sdu.edu.cn.
References
- 1.Clomburg JM, Gonzalez R. Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol. 2013;31:20–28. doi: 10.1016/j.tibtech.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Yazdani SS, Gonzalez R. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol. 2007;18:213–219. doi: 10.1016/j.copbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DT, Taconi KA. The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog. 2007;26:338–348. doi: 10.1002/ep.10225. [DOI] [Google Scholar]
- 4.Mattam AJ, Clomburg JM, Gonzalez R, Yazdani SS. Fermentation of glycerol and production of valuable chemical and biofuel molecules. Biotechnol Lett. 2013;35:831–842. doi: 10.1007/s10529-013-1240-4. [DOI] [PubMed] [Google Scholar]
- 5.Posada JA, Rincón LE, Cardona CA. Design and analysis of biorefineries based on raw glycerol: addressing the glycerol problem. Bioresour Technol. 2012;111:282–293. doi: 10.1016/j.biortech.2012.01.151. [DOI] [PubMed] [Google Scholar]
- 6.Katryniok B, Kimura H, Skrzyńska E, Girardon J-S, Fongarland P, Capron M, et al. Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem. 2011;13:1960. doi: 10.1039/c1gc15320j. [DOI] [Google Scholar]
- 7.Khanna S, Goyal A, Moholkar VS. Microbial conversion of glycerol: present status and future prospects. Crit Rev Biotechnol. 2012;32:235–262. doi: 10.3109/07388551.2011.604839. [DOI] [PubMed] [Google Scholar]
- 8.da Silva GP, Mack M, Contiero J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv. 2009;27:30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Nikel PI, Kim J, de Lorenzo V. Metabolic and regulatory rearrangements underlying glycerol metabolism in Pseudomonas putida KT2440. Environ Microbiol. 2014;16:239–254. doi: 10.1111/1462-2920.12224. [DOI] [PubMed] [Google Scholar]
- 10.Metsoviti M, Paraskevaidi K, Koutinas A, Zeng AP, Papanikolaou S. Production of 1,3-propanediol, 2,3-butanediol and ethanol by a newly isolated Klebsiella oxytoca strain growing on biodiesel-derived glycerol based media. Process Biochem. 2012;47:1872–1882. doi: 10.1016/j.procbio.2012.06.011. [DOI] [Google Scholar]
- 11.Yen HW, Li FT, Chang JS. The effects of dissolved oxygen level on the distribution of 1,3-propanediol and 2,3-butanediol produced from glycerol by an isolated indigenous Klebsiella sp. Ana-WS5. Bioresour Technol. 2014;153:374–378. doi: 10.1016/j.biortech.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Li Z, Shimizu K, Ye Q. Simultaneous production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol by a recombinant strain of Klebsiella pneumoniae. Bioresour Technol. 2012;103:351–359. doi: 10.1016/j.biortech.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Almeida JR, Fávaro LC, Quirino BF. Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol Biofuels. 2012;5:48. doi: 10.1186/1754-6834-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang MH, Jiang JG. Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog Lipid Res. 2013;52:395–408. doi: 10.1016/j.plipres.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Munch G, Sestric R, Sparling R, Levin DB, Cicek N. Lipid production in the under-characterized oleaginous yeasts, Rhodosporidium babjevae and Rhodosporidium diobovatum, from biodiesel-derived waste glycerol. Bioresour Technol. 2015;185:49–55. doi: 10.1016/j.biortech.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Zhu Z, Wang WH, Lu XF. Microbial recycling of glycerol to biodiesel. Bioresour Technol. 2013;150:1–8. doi: 10.1016/j.biortech.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 17.Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 18.Nikel PI, Martínez-García E, de Lorenzo V. Biotechnological domestication of pseudomonads using synthetic biology. Nat Rev Microbiol. 2014;12:368–379. doi: 10.1038/nrmicro3253. [DOI] [PubMed] [Google Scholar]
- 19.Gao C, Hu C, Zheng Z, Ma C, Jiang T, Dou P, et al. Lactate utilization is regulated by the FadR-type regulator LldR in Pseudomonas aeruginosa. J Bacteriol. 2012;194:2687–2692. doi: 10.1128/JB.06579-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiménez JI, Miñambres B, García JL, Díaz E. Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol. 2002;4:824–841. doi: 10.1046/j.1462-2920.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 21.Park SJ, Choi JS, Kim BC, Jho SW, Ryu JW, Park D, et al. PutidaNET: interactome database service and network analysis of Pseudomonas putida KT2440. BMC Genom. 2009;10(Suppl. 3):S18. doi: 10.1186/1471-2164-10-S3-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Lv M, Zhang Y, Xiao X, Jiang T, Zhang W, et al. Reconstruction of lactate utilization system in Pseudomonas putida KT2440: a novel biocatalyst for l-2-hydroxy-carboxylate production. Sci Rep. 2014;4:6939. doi: 10.1038/srep06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escapa IF, del Cerro C, García JL, Prieto MA. The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol. 2013;15:93–110. doi: 10.1111/j.1462-2920.2012.02790.x. [DOI] [PubMed] [Google Scholar]
- 24.Nikel PI, Romero-Campero FJ, Zeidman JA, Goñi-Moreno Á, de Lorenzo V. The glycerol-dependent metabolic persistence of Pseudomonas putida KT2440 reflects the regulatory logic of the GlpR repressor. mBio. 2015;6:e00340–15. [DOI] [PMC free article] [PubMed]
- 25.Zeng G, Ye S, Larson TJ. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: primary structure and identification of the DNA-binding domain. J Bacteriol. 1996;178:7080–7089. doi: 10.1128/jb.178.24.7080-7089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson TJ, Ye SZ, Weissenborn DL, Hoffmann HJ, Schweizer H. Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem. 1987;262:15869–15874. [PubMed] [Google Scholar]
- 27.Škerlová J, Fábry M, Hubálek M, Otwinowski Z, Rezáčová P. Structure of the effector-binding domain of deoxyribonucleoside regulator DeoR from Bacillus subtilis. FEBS J. 2014;281:4280–4292. doi: 10.1111/febs.12856. [DOI] [PubMed] [Google Scholar]
- 28.Gao C, Jiang T, Dou P, Ma C, Li L, Kong J, et al. NAD-independent l-lactate dehydrogenase is required for l-lactate utilization in Pseudomonas stutzeri SDM. PLoS One. 2012;7:e36519. doi: 10.1371/journal.pone.0036519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang T, Gao C, Su F, Zhang W, Hu C, Dou P, et al. Genome sequence of Pseudomonas stutzeri SDM-LAC, a typical strain for studying the molecular mechanism of lactate utilization. J Bacteriol. 2012;194:894–895. doi: 10.1128/JB.06478-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp MB. d- and l-Lactate dehydrogenases of Pseudomonas aeruginosa. Biochem J. 1972;130:307–309. doi: 10.1042/bj1300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Gao C, Qiu J, Hao J, Liu W, Wang A, et al. Membrane-bound l- and d-lactate dehydrogenase activities of a newly isolated Pseudomonas stutzeri strain. Appl Microbiol Biotechnol. 2007;77:91–98. doi: 10.1007/s00253-007-1132-4. [DOI] [PubMed] [Google Scholar]
- 32.Gao YG, Suzuki H, Itou H, Zhou Y, Tanaka Y, Wachi M, et al. Structural and functional characterization of the LldR from Corynebacterium glutamicum: a transcriptional repressor involved in l-lactate and sugar utilization. Nucleic Acids Res. 2008;36:7110–7123. doi: 10.1093/nar/gkn827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huffman JL, Brennan RG. Prokaryotic transcription regulators: more than just the helix-turn-helix motif. Curr Opin Struct Biol. 2002;12:98–106. doi: 10.1016/S0959-440X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 34.Jain D. Allosteric control of transcription in GntR family of transcription regulators: a structural overview. IUBMB Life. 2015;67:556–563. doi: 10.1002/iub.1401. [DOI] [PubMed] [Google Scholar]
- 35.Gao C, Xu X, Hu C, Zhang W, Zhang Y, Ma C, et al. Pyruvate producing biocatalyst with constitutive NAD-independent lactate dehydrogenases. Process Biochem. 2010;45:1912–1915. doi: 10.1016/j.procbio.2010.05.029. [DOI] [Google Scholar]
- 36.Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 37.Gao C, Qiu J, Ma C, Xu P. Efficient production of pyruvate from dl-lactate by the lactate-utilizing strain Pseudomonas stutzeri SDM. PLoS One. 2012;7:e40755. doi: 10.1371/journal.pone.0040755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VA, et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol. 2002;4:799–808. doi: 10.1046/j.1462-2920.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 39.Wakabayashi S, Matsubara H, Webster D. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature. 1986;322:481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- 40.Stark BC, Dikshit KL, Pagilla KR. The biochemistry of Vitreoscilla hemoglobin. Comput Struct Biotechnol J. 2012;3:1–8. doi: 10.5936/csbj.201210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khosla C, Bailey J. Heterologous expression of a bacterial hemoglobin improves the growth properties of recombinant Escherichia coli. Nature. 1988;331:633–635. doi: 10.1038/331633a0. [DOI] [PubMed] [Google Scholar]
- 42.Dikshit KL, Webster D. Cloning, characterization and expression of the bacterial globin gene from Vitreoscilla in Escherichia coli. Gene. 1988;70:377–386. doi: 10.1016/0378-1119(88)90209-0. [DOI] [PubMed] [Google Scholar]
- 43.Stark BC, Pagilla KR, Dikshit KL. Recent applications of Vitreoscilla hemoglobin technology in bioproduct synthesis and bioremediation. Appl Microbiol Biotechnol. 2015;99:1627–1636. doi: 10.1007/s00253-014-6350-y. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Tao F, Ma C, Xu P. New constitutive vectors: useful genetic engineering tools for biocatalysis. Appl Environ Microbiol. 2013;79:2836–2840. doi: 10.1128/AEM.03746-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang T, Gao C, Dou P, Ma C, Kong J, Xu P. Rationally re-designed mutation of NAD-independent l-lactate dehydrogenase: high optical resolution of racemic mandelic acid by the engineered Escherichia coli. Microb Cell Fact. 2012;11:151. doi: 10.1186/1475-2859-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma CQ, Xu P, Dou YM, Qu YB. Highly efficient conversion of lactate to pyruvate using whole cells of Acinetobacter sp. Biotechnol Prog. 2003;19:1672–1676. doi: 10.1021/bp0341242. [DOI] [PubMed] [Google Scholar]
- 47.Gao C, Zhang W, Lv C, Li L, Ma C, Hu C, et al. Efficient production of 2-oxobutyrate from 2-hydroxybutyrate by using whole cells of Pseudomonas stutzeri strain SDM. Appl Environ Microbiol. 2010;76:1679–1682. doi: 10.1128/AEM.02470-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta R, Henry M. Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol. 2006;81:1119–1129. doi: 10.1002/jctb.1486. [DOI] [Google Scholar]
- 49.Yang F, Hanna MA, Sun R. Value-added uses for crude glycerol–a byproduct of biodiesel production. Biotechnol Biofuels. 2012;5:13. doi: 10.1186/1754-6834-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu P, Qiu J, Gao C, Ma C. Biotechnological routes to pyruvate production. J Biosci Bioeng. 2008;105:169–175. doi: 10.1263/jbb.105.169. [DOI] [PubMed] [Google Scholar]
- 51.Koh-Banerjee PK, Ferreira MP, Greenwood M, Bowden RG, Cowan PN, Almada AL, et al. Effects of calcium pyruvate supplementation during training on body composition, exercise capacity, and metabolic responses to exercise. Nutrition. 2005;21:312–319. doi: 10.1016/j.nut.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 52.McCarty MF. Toward a wholly nutritional therapy for type 2 diabetes. Med Hypotheses. 2000;54:483–487. doi: 10.1054/mehy.1999.0881. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eggeling L, Morbach S, Sahm H. The fruits of molecular physiology: engineering the l-isoleucine biosynthesis pathway in Corynebacterium glutamicum. J Biotechnol. 1997;56:167–182. doi: 10.1016/S0168-1656(97)00115-6. [DOI] [Google Scholar]
- 55.Krix G, Bommarius AS, Drauz K, Kottenhahn M, Schwarm M, Kula MR. Enzymatic reduction of α-keto acids leading to l-amino acids, d- or l-hydroxy acids. J Biotechnol. 1997;53:29–39. doi: 10.1016/S0168-1656(96)01657-4. [DOI] [Google Scholar]
- 56.Atsumi S, Hanai T, Liao JC. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature. 2008;451:86–89. doi: 10.1038/nature06450. [DOI] [PubMed] [Google Scholar]
- 57.Zhang K, Li H, Cho KM, Liao JC. Expanding metabolism for total biosynthesis of the nonnatural amino acid l-homoalanine. Proc Natl Acad Sci USA. 2010;107:6234–6239. doi: 10.1073/pnas.0912903107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang W, Gao C, Che B, Ma C, Zheng Z, Qin T, et al. Efficient bioconversion of l-threonine to 2-oxobutyrate using whole cells of Pseudomonas stutzeri SDM. Bioresour Technol. 2012;110:719–722. doi: 10.1016/j.biortech.2012.01.123. [DOI] [PubMed] [Google Scholar]
- 59.Miller JH. A short course in bacterial genetics: A laboratory manual and handbook for Escherichia coli and related bacteria. New York: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 60.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 61.Armstrong JM. The molar extinction coefficient of 2,6-dichlorophenol Indophenol. Biochim Biophys Acta. 1964;86:194–197. doi: 10.1016/0304-4165(64)90180-1. [DOI] [PubMed] [Google Scholar]


