Fig. 1.
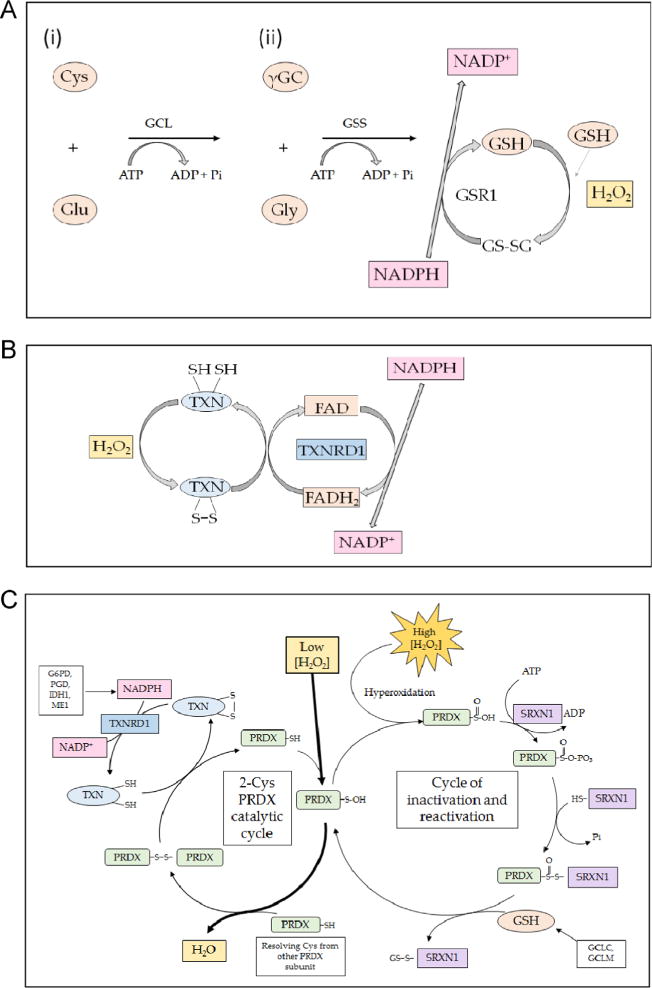
Mechanism of action of endogenous small protein antioxidants. (A) The biosynthesis of glutathione involves two steps. First (i), glutamate-cysteine ligase (GCL) conjugates cysteine (Cys) with glutamate (Glu), in a rate-limiting reaction that requires ATP, to produce γ-glutamyl-cysteine (γGC). Second (ii), glutathione synthetase (GSS) attaches glycine (Gly) to the C-terminal cysteine of γGC to produce the tripeptide glutathione (GSH). In turn, GSH may be oxidized by ROS (shown as H2O2), generating a disulfide bridge between two glutathione molecules, resulting in the formation of GSSG. The GSSG can be reduced back to two GSH molecules by the action of GSR1, an enzyme that utilizes NADPH as the electron donor. (B) The small protein dithiol TXN is oxidized, producing an intermolecular disulfide that is reduced by the flavoprotein TXNRD1, using NADPH as the electron donor. (C) The typical 2-Cys PRDX isoenzymes 1, 2, 3, and 4 reduce H2O2 through a catalytic cycle (shown on the left-hand side) that involves oxidation of the – SH group in an active-site Cys residue to sulfenic acid (–S–OH) in one subunit of the dimeric proteins. The oxidized thiol then forms an intermolecular disulfide bridge with a Cys residue in the other subunit before it is reduced by TXN; the resulting oxidized TXN is reduced by TXNRD1 in an NADPH-dependent manner. During reduction of H2O2, the active-site Cys in a small fraction of PRDX is hyperoxidized to sulfinic acid (–SO2H) (shown on the right-hand side). Overoxidation of the peroxidatic Cys to sulfinic acid inactivates PRDX, but it can be reactivated by SRXN1 through a mechanism that involves a transient covalent linkage between the two proteins, followed by a thiol-mediated reduction that is likely to involve GSH.
