Fig. 9.
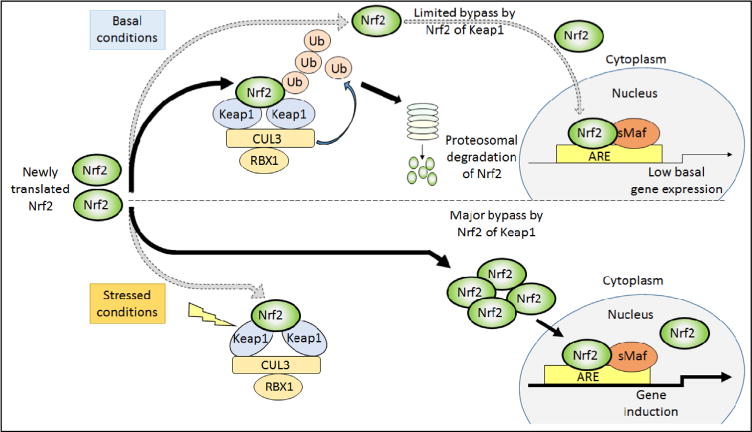
Regulation of Nrf2 by Keap1 under basal and stressed conditions. Under unstressed conditions, newly synthesized Nrf2 protein is short lived, being sequestered in the cytoplasm by Keap1 and targeted immediately for proteasomal degradation by CRLKeap1. Under such circumstances, Nrf2 is rapidly ubiquitylated and degraded, leaving the Keap1 homodimer free to sequester and process newly translated Nrf2 for degradation. A small fraction of the Nrf2 escapes degradation and translocates to the nucleus, maintaining a low basal level of ARE-driven gene expression even under normal homeostatic circumstances. Under stressed conditions, modification of Keap1 causes a conformational change that prevents the CRLKeap1 protein complex from ubiquitylating Nrf2. In this situation, Keap1 is unable to turn over bound Nrf2, and as “free-Keap1” cannot be regenerated, it is unable to bind newly translated Nrf2. Thus under stressed conditions, newly synthesized Nrf2 evades Keap1 capture and translocates directly to the nucleus where it heterodimerizes with sMaf proteins, and together they bind ARE sequences to transactivate target genes.
