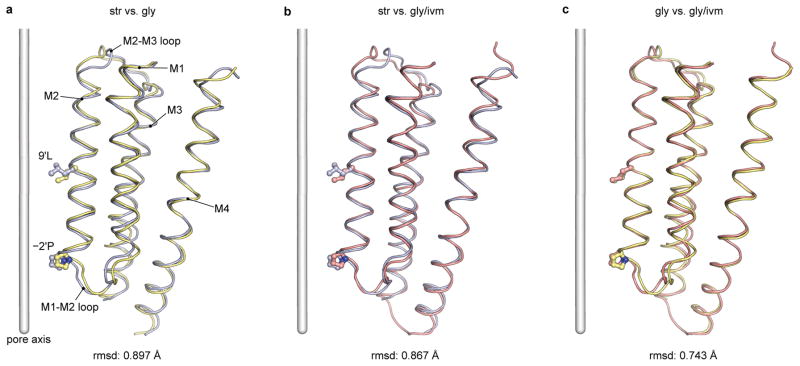
Extended Data Figure 8. Superimposition of TMD between str- and gly-GlyR (a), str- and gly/ivm-GlyR (b), gly- and gly/ivm-GlyR (c) using main chain atoms of residues Met236-Lys362.
The M2-M3 loop, residues Ser289-Ala298, is excluded from the comparison. The r.m.s.d. are 0.9, 0.9 and 0.7 A, respectively, suggesting that the movement of the TMD is rigid-body-like. Most differences are located in the termini of transmembrane helices, which are either close to the M2-M3 loop, or close to the intracellular gate −2′Pro.