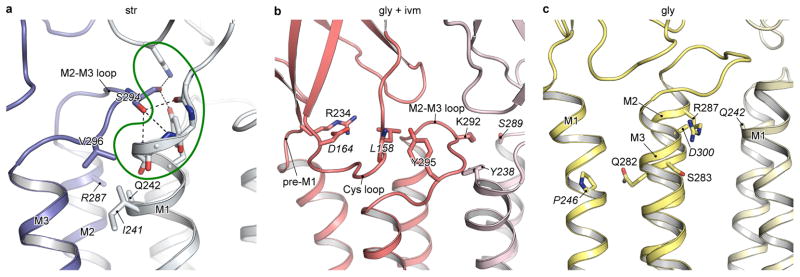
Extended Data Figure 9. Positions of residues whose mutations are associated with human startle disease.
Residues that likely interact with disease-causing residues are labeled in italics. a, The str-GlyR model is used to show residues whose mutations cause spontaneous activation. The mutation of Gln242 in M1 to glutamate may enhance its electrostatic attraction to Arg287 in M2 of the adjacent subunit, tilt the upper part of M2 away from pore axis, resulting in a constitutively open channel. On the other hand, the mutation Val296Met in M2-M3 loop may cause steric collision with Ile241 in M1 of the adjacent subunit, and prevent Ser294 from interacting to the N-cap formed by pre-M1, M1 and the β8-β9 loop, thereby destabilizing the closed conformation. b, The gly/ivm-GlyR model is used to show residues in the ECD-TMD interface whose mutations reduce sensitivity to glycine and single channel conductance. The mutation of Arg234 in pre-M1 to glutamine may disturb its electrostatic interaction with Asp164 in the Cys-loop. Similarly, the mutation of Tyr295 in the M2-M3 loop to cysteine or serine may disturb its interaction with the main chain nitrogen atom of Leu158 in the Cys-loop. In both cases, the signal induced by agonist binding may be blocked. The mutation Lys292Glu in the M2-M3 loop possibly affects the cooperative interaction between two adjacent subunits by altering the van der Waals contacts between Lys292 and Tyr238. c, The gly-GlyR model is used to show residues in M2 whose mutations reduce sensitivity to glycine and diminish single channel conductance. These mutations may directly influence the pore properties by modifying the interactions with adjacent residues, for instance, between Gln282His and Pro246, and between Arg287Gln/Leu and Gln242.