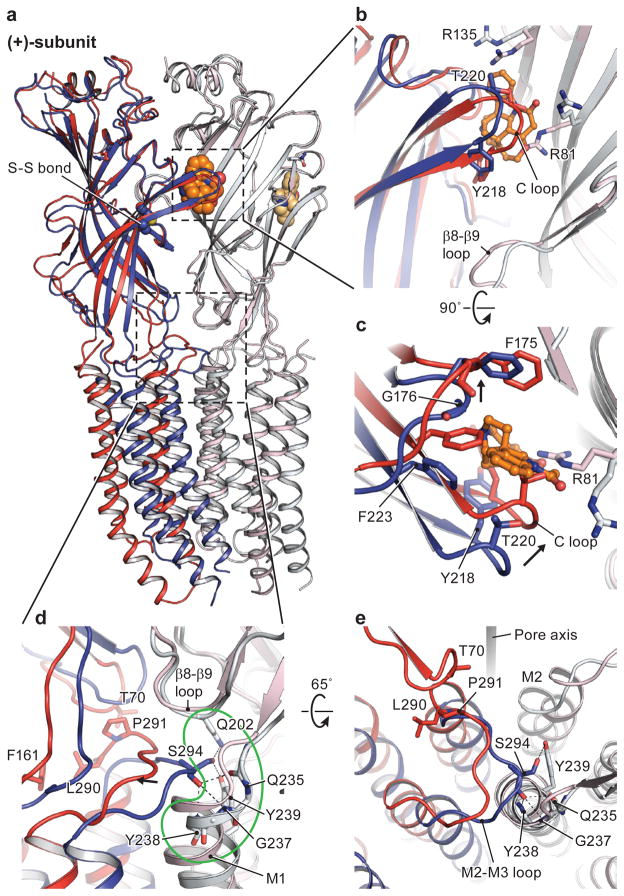
Figure 5. Conformational differences at the subunit-subunit interface between agonist- and antagonist-bound states.
Str- and gly/ivm-bound states are in blue and red, respectively. The (−)subunits are in corresponding light colors. a, Superimposition of the ECD of the (−)-subunits showing the relative movement of the (+)-subunits. In b and c are shown conformational changes of the neurotransmitter binding pocket, viewed parallel to the membrane and from the extracellular side, respectively. The neurotransmitter binding site expands in the str-bound structure caused by repositioning of Arg135 and Arg81 in the (−)-subunit and by the opening of the C loop in the (+)subunit. Panels d and e illustrate the coupling of structural rearrangements of the ECD-TMD interface between two adjacent subunits. In the str-bound form, Ser294 in the M2-M3 loop of the (+)-subunit is inserted in the M1 N-cap in the (−)-subunit. Key residues interacting with Ser294 are highlighted in the green outline. Upon binding of glycine, the M2-M3 loop moves away from the N-cap. For clarity, the side chains of Gln235 and Tyr238 are not shown.