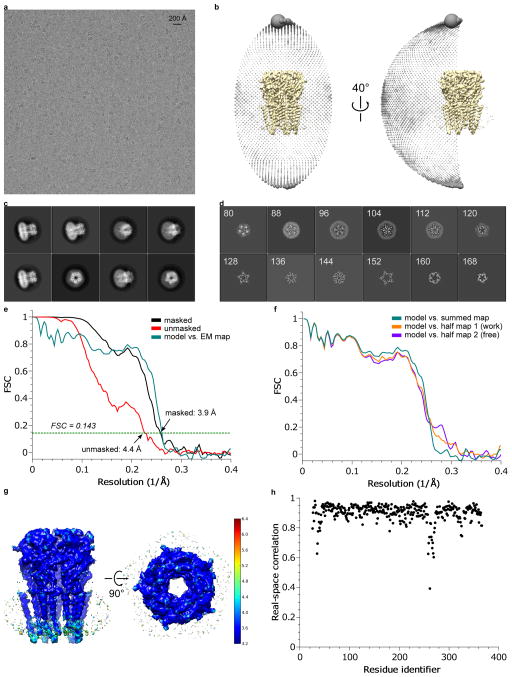
Extended Data Figure 2. 3D reconstruction of glycine-bound GlyR.
a, A representative micrograph (out of 1460 migrographs) of gly-bound GlyR in vitreous ice. b, Angular distribution of particle projections, and c, selected 2D classes are shown. In panel (c), the radius of the sphere is proportional to the number of particles assigned to it. The plot is drawn with respect to the 3D reconstruction shown in the center, taking the C5 symmetry of the receptor into account. d, Selected ‘slice’ views of the final reconstruction along the pore axis. The slice numbers are indicated, starting from the intracellular side. e, FSC curves for the density maps before (red) and after (black) post-processing in RELION. The FSC curve between the refined atomic model and the final reconstruction map is shown in green. f, FSC curves for cross-validation: model versus summed map (full data set, green), model versus half map 1 (used in test refinement, orange) and model versus half map 2 (not used in test refinement, blue). g, Unfiltered and unsharpened 3D density map colored according to local resolution estimated using RESMAP. h, Real-space correlation between atomic model and density map calculated using PHENIX.