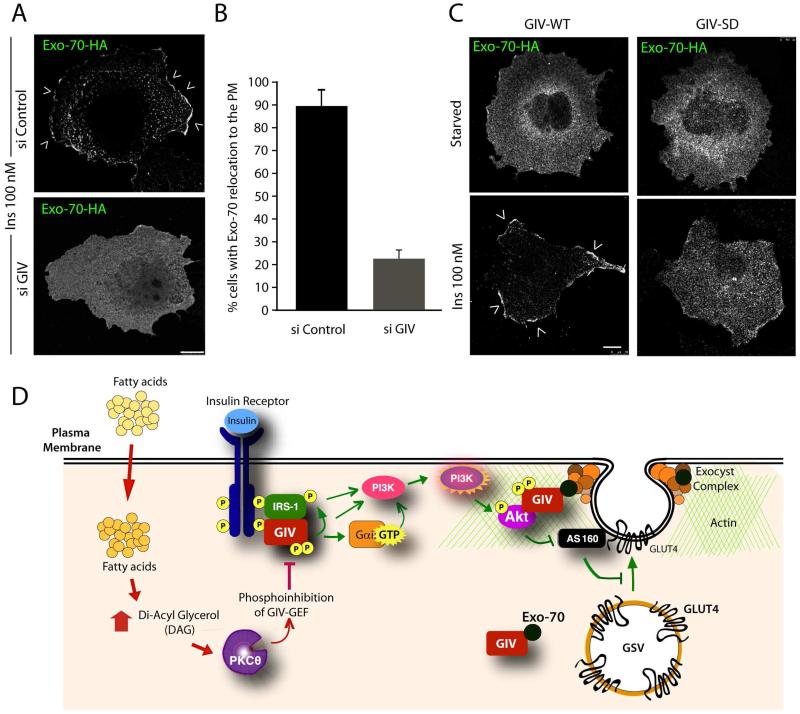
Figure 4. GIV and its GEF function are required for the relocation of Exo-70 to the PM.
(A) Control (si Control) or GIV-depleted (si GIV) expressing Exo-70-HA were serum starved and subsequently stimulated with insulin for 10 min prior to fixation. Fixed cells were stained with HA (green; shown here in grayscale). Arrowheads = PM. Bar = 10 μm. (B) Bar graph displays the % cells in A in which Exo-70 was translocated to the PM. p = 0.0001. (C) Cos7 cells coexpressing Exo-70-HA and either GIV-WT-FLAG or GIV-SD-FLAG were serum starved and insulin-stimulated and then stained with HA (green; shown here in grayscale). FLAG (red) and DNA/DAPI (blue) are displayed in Figure S4. Arrowheads = PM. Bar = 10 μm. (D) Schematic summarizing our proposed model for how GIV interacts with several key upstream and downstream components of rapid insulin response (Akt, actin, GCVs, exo70) that are essential for efficient GLUT4 exocytosis and glucose uptake in response to insulin. Previous work [3] has shown that physiologic insulin response (pathways indicated in green on the right) requires insulin triggered formation of InsR-GIV complexes, followed by tyrosine phosphorylation of GIV (pY1764), and subsequent activation of Gαi via GIV’s GEF function. Consequently, metabolic insulin signaling is enhanced through the InsR/IRS1/PI3K/Akt pathway, which culminates in phosphoinhibition of RabGAP AS160. Findings in this work indicate that GIV associates constitutively with Exo-70 subunit of the exocyst complex, and that it associates also with GSVs exclusively after ligand stimulation. In doing so, GIV anatomically couples the signaling components (InsR, IRS1, Akt and PI3K) to the membrane trafficking components (AS160, GSVs, the exocyst complex) that regulate GSV exocytosis for rapid uptake of glucose. In IR (pathways shown in red on the left), circulating free fatty-acids trigger the accumulation of diacyl glycerol (DAG) and PKCθ is activated. PKCθ phosphorylates GIV at S1689 and turns “off” its GEF function. Consequently, Gαi remains inactive and the InsR/IRS1/PI3K/Akt/AS160 signaling cascade is suppressed, Exo-70 fails to translocate to the PM, and GSV exocytosis and glucose uptake are impaired.