Abstract
Background
Neurofibromatosis type 1 (NF1) is a common, autosomal dominant tumor-predisposition disorder that arises secondary to mutations in the tumor suppressor gene NF1. Cephalometry is an inexpensive, readily available and non-invasive technique that is under-utilized in studying the NF1 craniofacial phenotype. An analysis of NF1 cephalometry was first published by Heervä et al. in 2011. We expand here on that first investigation with a larger cohort of adult and pediatric patients affected with NF1 and sought objective insight into the NF1 facies, said to feature hypertelorism and a broad nasal base, from cephalometric analysis.
Methods
We obtained cephalograms from 101 patients with NF1 (78 adults and 23 children) from two NF1 protocols at the National Institutes of Health. Each subject had an age-, gender- and ethnicity-matched control. We used Dolphin software to make the cephalometric measurements. We assessed the normality of differences between paired samples using the Shapiro-Wilk test and evaluated the significance of mean differences using paired t-tests and adjusted for multiple testing. We explored the relationship between the cephalometric measurements and height, head circumference and interpupillary distance.
Results
In this dataset of American whites with NF1, we confirmed in a modestly larger sample many of the findings found by Heerva et al. in an NF1 Finnish cohort. We found a shorter maxilla, mandible, cranial base, (especially anteriorly, p = 0.0001) and diminished facial height in adults, but not children, with NF1. Only one adult exhibited hypertelorism.
Conclusions
The cephalometric differences in adults arise in part from cranial base shortening and thus result in a shorter face, mid-face hypoplasia, reduced facial projection, smaller jaw, and increased braincase globularity. In addition, we suggest that NF1 sphenoid bone shortening, a common event, is consistent with an intrinsic NF1 bone cell defect, which renders the bone more vulnerable to a random “second hit” in NF1, leading to sphenoid wing dysplasia, a rare event.
Keywords: Neurofibromatosis type 1, cephalometery, dysmorphology, sphenoid wing dysplasia
Background
Neurofibromatosis type 1 (NF1, also known as von Recklinghausen’s disease) is a genetic disorder with an autosomal dominant pattern of inheritance affecting the skin, skeletal, and neural tissues, and affects 1 in 3000 births with no gender or race predilection [1]. Individuals with NF1 are heterozygous (haploinsufficient) for a loss-of-function mutation in NF1, the gene that encodes the tumor suppressor neurofibromin, which negatively regulates the activity of the intracellular signaling molecule Ras by acting as a GTPase activating protein (Ras-GAP). At a cellular level, this results in hyperactivation of the Ras pathway, which in turn activates a variety of signaling pathways in a broad range of cells and tissue types [2], including bone and cartilage. In particular, neurofibromin functions in osteoblast differentiation and mineralization [3].
Consequently, the clinical phenotype of NF1 is manifested by a wide variability in multiple organ systems. Common signs of NF1 include multiple café-au-lait spots, axillary and inguinal freckling, multiple discrete dermal neurofibromas, learning disabilities, Lisch nodules, and an increased risk of a variety of benign and malignant tumors. Dental and jaw abnormalities including increased caries, premature tooth eruption, an enlarged mandibular foramen, a wide and branching inferior alveolar canal, and periapical cemental dysplasia have been reported [4]. Skeletal abnormalities occur in nearly half of NF1 patients. These include focal bony lesions, mild shortness of stature, tibial dyplasia, scoliosis, and sphenoid wing dysplasia [5]. Sphenoid wing dysplasia, almost always unilateral, is a cranial defect unique to NF1 and is rare even within the NF1 patient population, typically presenting before age two years. In some cases it can arise as a secondary response to an adjacent soft tissue abnormality like a plexiform neurofibroma, a type of congenital neurofibroma [6, 7]. However, it is also hypothesized to develop due to a primary bone cell-autonomous defect [5]. An analysis of NF1 cephalometry was first published by Heervä et al. in 2011, who found that individuals with NF1 typically had a shorter mandible, maxilla and cranial base compared to controls [8]. The sphenoid bone is a key component of the anterior cranial base. We expand here on that first investigation with a larger cohort of adult and pediatric patients affected with NF1 and focused on the cephalometric effects of NF1 haploinsufficiency on the cranial base, and the sphenoid bone in particular.
Methods
Retrospective ascertainment of patients and phenotype data
A total of 80 adults were included in the study, but two adult patient-control pairs were excluded due to anomalously low measurements, yielding a total of 78 adult case-control pairs for our final analysis. These patients were diagnosed with NF1 per the NIH consensus criteria [9] and evaluated at the National Institutes of Health (NIH) in Bethesda, MD as part of the protocol “Variation in Gene Expression in Neurofibromatosis Type 1” (05-HG-0152) to quantitatively phenotype and identify genetic modifiers of severity in NF1. We obtained records from all patients from the study evaluated at the dental clinic. Measurements of height, head circumference and interpupillary distance were available for all adult subjects. Height centile was determined against a normal reference population (CDC 2000 growth charts) and against an NF1 reference population [10]; head circumference was determined against a normal reference after adjustment for height and gender [11]. We estimated IPD from the inner and outer canthal distances according to Pryor’s method [12]. We compared these estimates of IPD to the standards for Caucasian males and females ages 16–24 published by Pryor and confirmed in Dodgson [13]. Interpupillary distance was calculated from measured inner and outer canthal distances on adult subjects, subtracted from population norms, and divided by standard deviations to yield standard deviation scores (SDS).
Hypertelorism was defined as having an IPD two standard deviations greater than the reference mean. A total of 23 children with NF1 from the “Natural History Study and Longitudinal Assessment of Children, Adolescents, and Adults with Neurofibromatosis Type 1” (08-C-0079) with lateral cephalometric measurements were also included. We obtained records from all patients from the study evaluated at the dental clinic. Height and head circumference data was available for the pediatric cases. We reviewed all patient records for documentation of known sphenoid wing dysplasia and/or tibial or ulnar dysplasias. Seventy-eight adult and 23 pediatric age-, gender- and ethnicity-matched controls were selected from a group of healthy patients with no history of genetic disorders, who had undergone orthodontic evaluation in the Department of Orthodontics, University of Maryland (UMB) in Baltimore, MD. Age matching was within one year for the pediatric cohort and three years for the adult cohort. This study was approved by the University of Maryland School of Dentistry and National Cancer Institute institutional review boards and all participants (or their parents or guardians) provided written, informed consent.
Cephalometric analysis
For all subjects, cephalometric radiographs were obtained from the National Institutes of Health and UMB for comparative analysis. All of the subjects’ identities were blinded to the investigator. Cephalometric radiographs were coded in a random order. Cephalometric radiographs were then digitally traced for landmarks and measurements by a single, different investigator for the pediatric and adult cohorts (Figure 1, Table 1). Sixteen linear and angular measurements were measured to the nearest tenth of a millimeter or degree, respectively, utilizing Dolphin software (version 11.5) (Dolphin Imaging, Chatsworth, CA).
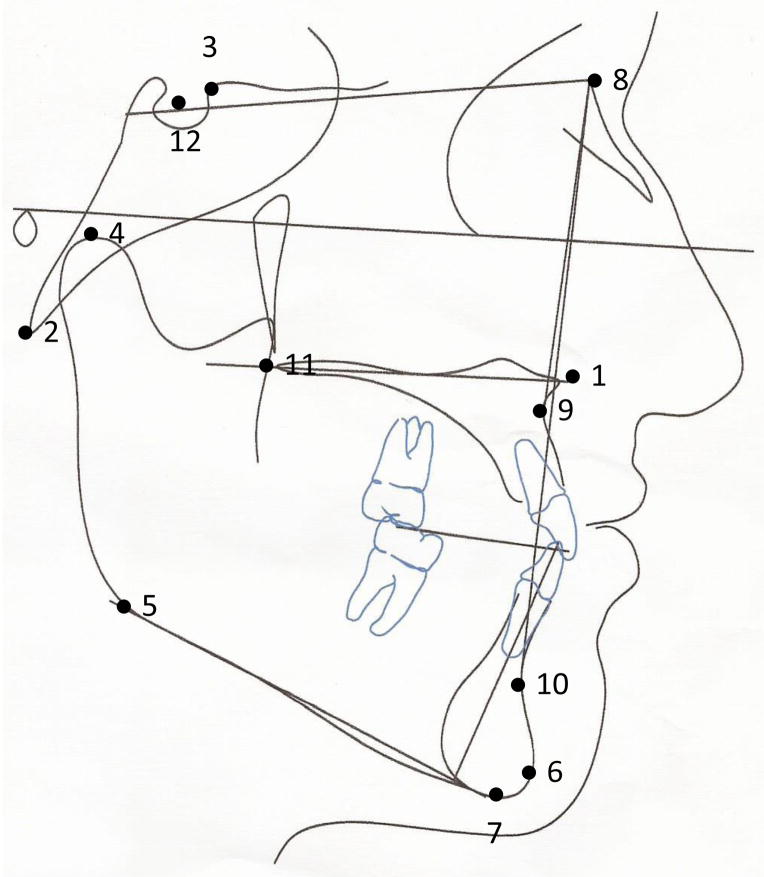
Figure 1.
- ANS (Anterior Nasal Spine): tip of the anterior nasal spine
- Ba (Basion): most postero-inferior point of the basilar part of the occipital bone
- Cl (Clinoidale): most superior point on the contour of the anterior clinoid
- Co (Condylion): most superior point of the mandibular condyle
- Go (Gonion): the most inferior, posterior, and lateral point on the angle of the mandible
- Gn (Gnathion): most antero-inferior midline point on the mandible
- M (Menton): most inferior point of the mandibular symphysis
- N (Nasion): most anterior point on the frontonasal suture
- A (Point A): deepest concavity of the anterior bony outline of the maxilla
- B (Point B): deepest concavity of the anterior bony outline of the mandible
- PNS (Posterior Nasal Spine): tip of the posterior spine of the palatine bone in the hard palate
- S (Sella): midpoint of sella turcica (not depicted)
Table 1.
Definition of Skeletal Measurements
| Vertical Facial Proportions |
| AFH: Anterior Facial Height, measured from Nasion-Menton, denotes length of anterior face |
| PFH: Posterior Facial Height, measured from Sella-Gonion, denotes length of posterior face |
| PFH/AFH: ratio of Posterior Facial Height to Anterior Facial Height; denotes differential length of anterior and posterior face |
| UAFH: Upper Anterior Facial Height, measured from Nasion-ANS |
| LAFH: Lower Anterior Facial Height, measured from ANS-Menton |
| LAFH/TAFH: ratio of Lower Anterior Facial Height to Total Facial Height; denotes proportion of lower anterior face. |
| Maxilla |
| PNS-ANS: maxillary length, distance measured from the anterior nasal spine to posterior nasal spine. |
| SNA: angle measured between Sella-Nasion and A point, denotes sagittal relationship of the maxilla to cranial base |
| ANB: angle measured between maxilla and mandible with respect to N, denotes the sagittal relationship between maxilla and the mandible |
| Mandible |
| Co-Gn: measured from condylion to gnathion in mm, denotes total mandibular length |
| Co-Go: condylion to gonial angle measured in mm, denotes the height of ramus of mandible |
| Sn-GoGn: measured as angle formed by S-N and lower border of the mandible(GoGn), denotes vertical relationship of the mandible to the cranial base. |
| SNB: angle measured between S-N and B point, denotes sagittal relationship of the mandible to cranial base |
| Cranial Base |
| S-N: measured from Nasion to Sella in mm, denotes length of anterior cranial base |
| S-Ba: measured from Sella-to Basion in mm, denotes length of posterior cranial base |
| Ba-S-N: cranial base angle formed by Nasion-Sella-Basion, denotes cranial base flexure |
Statistical analysis
We assessed the normality of differences between paired samples using the Shapiro-Wilk test and evaluated the significance of mean differences using paired t-tests. For two adult (LAFH and S-Ba) and three pediatric (UAFH, Sn-Go-Gn and Ba-S-N) cephalometric measures, the differences were not normally distributed and we instead used the Wilcoxon matched-pairs signed-rank test. For each of the 16 cephalometric measurements in the separate adult and pediatric cohorts, we calculated differences for the whole sample and by gender. We adjusted for multiple comparisons using a Bonferroni correction; considering our 16 cephalometric measurements, a p-value < 0.003 (= 0.05/16) was deemed statistically significant. We used the Wilcoxon rank-sum test to assess whether the effect of NF1 on cephalometric measurements differed between genders. We calculated Pearson correlation coefficients for the cephalometric measurements and height and head circumference (71 adults; seven without these measurements), and interpupillary distance (64 adults; fourteen without this measurement). All 23 pediatric NF1 patients had height and head circumference measurements, but none had inner canthal, outer canthal or interpupillary distance measurements. We considered p-values below 0.05 to be statistically significant. Statistical analyses were performed using IBM SPSS Base 20.0 (IBM Corporation, Armonk, NY) and Stata/SE 13.1 (Stata Corp LP, College Station, TX).
Results
Patient demographics
Patient characteristics are summarized in Table 2. In the adult study, there were 33 males (42%) and 45 females (58%). In the pediatric study, there were 14 males (61%) and 9 females (39%). All subjects were white.
Table 2.
Age distribution in cases and controls.
| Mean ± SD | ||
|---|---|---|
| NF1 | Control | |
| Age 18+ (n=78) | 38.3 ± 16 | 38.4 ± 15.7 |
| Males (n=33) | 35.6 ± 15.8 | 35.9 ± 15.3 |
| Females (n=45) | 40 ± 16.1 | 39.9 ± 16 |
| Age <18 (n=23) | 12.2 ± 2.9 | 12.3 ± 3.1 |
| Males (n=14) | 11.9 ± 2.3 | 12.2 ± 2.7 |
| Females (n=9) | 12.7 ± 3.8 | 12.3 ± 3.8 |
Cephalometric analyses in cases and controls
Analyses of difference in 16 cephalometric measurements between cases and controls are shown in Table 3 (adults) and in Table 4 (children); these analyses stratified by gender are shown in Supplemental Tables 1 and 2 for adults and children, respectively.
Table 3.
Difference between matched cases and controls age 18+ yrs.
| n=78 | Cephalometric measurement | Control | NF1 | Mean Diff. | p-value |
|---|---|---|---|---|---|
| Vertical | AFH | 124 ± 16.3 | 115.5 ± 8.98 | 8.48 | 0.0001 |
| Vertical | PFH | 79.3 ± 9.25 | 77.2 ± 7.85 | 2.17 | 0.0777 |
| Vertical | PFH/AFH | 0.64 ± 0.07 | 0.67 ± 0.05 | −0.02 | 0.0166 |
| Vertical | UAFH | 53.8 ± 3.99 | 51.7 ± 4.36 | 2.11 | 0.0026 |
| Vertical | LAFH | 67.5 ± 6.54 | 64.1 ± 7.03 | 3.36 | 0.0002* |
| Vertical | LAFH/AFH | 0.56 ± 0.02 | 0.55 ± 0.03 | 0 | 0.4595* |
|
| |||||
| Maxillary | ANS-PNS | 50.3 ± 4.64 | 47.8 ± 3.84 | 2.5 | 0.0004 |
| Maxillary | SNA | 81 ± 4.31 | 81.2 ± 4.02 | −0.22 | 0.747 |
| Maxillary | ANB | 3.64 ± 2.91 | 2.18 ± 2.66 | 1.46 | 0.0009 |
|
| |||||
| Mandibular | Co-Gn | 119.8 ± 8.93 | 116.3 ± 7.96 | 3.57 | 0.0049 |
| Mandibular | Co-Go | 58.5 ± 6.89 | 55.8 ± 6.86 | 2.65 | 0.0136 |
| Mandibular | Sn-Go-Gn | 31.14 ± 7.19 | 29.2 ± 6.41 | 1.92 | 0.0713 |
| Mandibular | SNB | 77.4 ± 4.38 | 79.1 ± 4.28 | −1.69 | 0.0144 |
|
| |||||
| Cranial Base | S-N | 72 ± 5.48 | 68.7 ± 4.48 | 3.28 | 0.0001 |
| Cranial Base | S-Ba | 45.7 ± 5.14 | 44 ± 5.84 | 1.68 | 0.0011* |
| Cranial Base | Ba-S-N | 127.6 ± 5.03 | 130.7 ± 6.9 | −3.08 | 0.0006 |
indicates data were not normally distributed and p-value is for signed-rank test; bolded values are significant after Bonferroni correction
Table 4.
Difference between matched cases and controls age <18 yrs.
| n=23 | Cephalometric measurement | Control | NF1 | Mean Diff. | p-value |
|---|---|---|---|---|---|
| Vertical | AFH | 108.3 ± 8.3 | 108.7 ± 9.4 | −0.41 | 0.8421 |
| Vertical | PFH | 71.2 ± 6.5 | 70.9 ± 8.6 | 0.26 | 0.8967 |
| Vertical | PFH/AFH | 0.66 ± 0.03 | 0.7 ± 0.05 | 0 | 0.7451 |
| Vertical | UAFH | 48.5 ± 4 | 47.8 ± 3.7 | 0.73 | 0.4029* |
| Vertical | LAFH | 61.4 ± 5.3 | 63.1 ± 6.8 | −1.64 | 0.295 |
| Vertical | LAFH/AFH | 0.56 ± 0.02 | 0.57 ± 0.02 | −0.01 | 0.0489 |
|
| |||||
| Maxillary | ANS-PNS | 45 ± 4.6 | 43.9 ± 5 | 1.08 | 0.3833 |
| Maxillary | SNA | 79.7 ± 8.3 | 81 ± 4.8 | −1.35 | 0.5293 |
| Maxillary | ANB | 2.6 ± 2.3 | 3.5 ± 3.3 | −0.99 | 0.184 |
|
| |||||
| Mandibular | Co-Gn | 109.6 ± 7.3 | 106.4 ± 11.1 | 3.26 | 0.154 |
| Mandibular | Co-Go | 52 ± 4.5 | 49.7 ± 5.9 | 2.25 | 0.1117 |
| Mandibular | Sn-Go-Gn | 31 ± 4.2 | 31.5 ± 5.5 | −0.55 | 0.254* |
| Mandibular | SNB | 78.4 ± 3.4 | 77.5 ± 4.7 | 0.9 | 0.5168 |
|
| |||||
| Cranial Base | S-N | 67 ± 3.9 | 65.7 ± 5.2 | 1.27 | 0.2712 |
| Cranial Base | S-Ba | 43.6 ± 3 | 42.5 ± 4.8 | 1.16 | 0.3516 |
| Cranial Base | Ba-S-N | 129.5 ± 4.8 | 133.4 ± 8.9 | −3.93 | 0.0463* |
indicates data were not normally distributed and p-value is for signed-rank test; bolded values are significant after Bonferroni correction
In adults, anterior facial height (AFH) was significantly reduced in NF1 cases and the difference persisted when the analysis was stratified by gender. AFH is the sum of the upper AFH (UAFH: from nasion to anterior nasal spine (ANS)) and lower AFH (LAFH: from ANS to menton); both UAFH and LAFH were significantly reduced in the entire cohort. Posterior facial height (PFH) was also reduced, but not significantly, in the entire cohort and in males. In the pediatric analysis of facial height, AFH, LAFH and UAFH were not significantly different between cases and controls.
In adults, the maxillary length (distance from ANS to the posterior nasal spine (PNS)) was significantly shortened in the entire cohort. This shortening was present in both males and females although not significantly when corrected for multiple testing. There were no significant changes in the sagittal relationship of the maxilla to the cranial base (SNA). The sagittal relationship of the maxilla and mandible (ANB) was significantly reduced in the entire cohort; these differences persisted in males and females, but were not significant when corrected for multiple testing. In children, no significant differences in ANS-PNS, SNA or ANB were observed.
The mandible overall was smaller in adults with NF1. The mandibular length (Co-Gn) was significantly shorter in NF1 and these differences were observed in both males and females, although not significantly after multiple testing correction. Ramal height (Co-Go), mandibular angle (Sn-Go-Gn) and the sagittal relationship of the mandible to the cranial base (SNB) were all smaller in NF1 individuals although not significantly after multiple testing correction. In children, no significant differences in these mandibular measurements were observed.
In adults, the anterior cranial base (S-N) was significantly shorter in individuals with NF1. This shortening was also observed in both males and females although not significantly in males after Bonferroni correction. Cranial base angle (S-N-Ba) was also significantly greater in NF1 compared with controls in the entire cohort. The posterior cranial base (S-Ba) was significantly shorter in NF1 cases; these differences persisted in males and females, although not significantly. In children, cranial base angle was also greater in NF1, but the difference was not significant after Bonferroni correction; neither the anterior nor the posterior cranial bases were significantly different relative to controls.
Correlation of cephalometric measurements with height, head circumference and interpupillary distance in NF1 patients
Supplemental Table 3 shows the centile distribution of height, occipital-frontal circumference (OFC; head circumference) and interpupillary distance (IPD; adults only) in NF1 cases; these data were not available for controls. In general, we observed the short stature and enlarged OFC typical of the NF1 population. Only one adult with NF1 was hyperteloric at examination (Table S3). Correlation coefficients of the 16 cephalometric measurements with height, OFC and IPD in adults and children are shown in Table 5. Numerous measurements, especially facial height, mandibular length and anterior cranial base length, were positively correlated with height in both the pediatric and adult populations. Post hoc analysis revealed that the adult cases who were below the 20th centile (n=34) for height had significantly shorter measures of facial height, while those above the 20th centile (n=44) were no different than their matched controls, suggesting that differences in height between cases and controls may explain differences in facial height observed between NF1 cases and controls. To further explore this finding, we repeated our cephalometric analyses stratified at the 20th centile of non-NF1 population height (Supplemental Table 4). By doing this, we could examine cephalometric measures in shorter (< 20th centile) and taller (≥ 20th centile) people with NF1. In NF1 cases < 20th centile for height, AFH, LAFH and Co-Gn (mandibular length) were all significantly shorter. The shorter anterior cranial base (S-N) was of borderline significance; many other measurements were shorter without adjustment for multiple comparisons. In NF1 cases ≥ 20th centile for height, only ANB (maxillary sagittal relationship to mandible) was significantly smaller; all other measurements were similar between cases and controls.
Table 5.
Correlation of 16 measurements with height, OFC, and IPD in adults and children
| Adults (age 18+ yrs) | Pediatric cases (<18 yrs) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Height (cm) (n=71) | OFC (cm) (n=71) | IPD (cm) (n=64) | Height (cm) (n=23) | OFC (cm) (n=23) | ||
| Vertical | AFH | 0.4939*** | 0.1284 | 0.2071 | 0.8036*** | 0.8442*** |
| Vertical | PFH | 0.5443*** | 0.3621** | 0.1750 | 0.759*** | 0.6617*** |
| Vertical | PFH/AFH | 0.226 | 0.3189** | 0.0356 | 0.2875 | 0.1256 |
| Vertical | UAFH | 0.2935* | 0.259* | 0.1904 | 0.6937*** | 0.7837*** |
| Vertical | LAFH | 0.4563*** | −0.02 | 0.1480 | 0.7182*** | 0.773*** |
| Vertical | LAFH/TAFH | 0.2222 | −0.1689 | −0.0196 | 0.3396 | 0.3107 |
|
| ||||||
| Maxillary | ANS-PNS | 0.2003 | 0.0161 | 0.0331 | 0.6001** | 0.4804* |
| Maxillary | SNA | 0.0551 | −0.1033 | −0.1449 | 0.3015 | 0.0253 |
| Maxillary | ANB | −0.1258 | −0.2064 | −0.1598 | −0.1707 | −0.0884 |
|
| ||||||
| Mandibular | Co-Gn | 0.524*** | 0.229 | 0.1210 | 0.814*** | 0.68*** |
| Mandibular | Co-Go | 0.4808*** | 0.2992* | 0.1247 | 0.6517*** | 0.5909** |
| Mandibular | Sn-Go-Gn | −0.096 | −0.2978* | −0.0040 | −0.3043 | −0.1235 |
| Mandibular | SNB | 0.1318 | 0.0349 | −0.0305 | 0.4327* | 0.0888 |
|
| ||||||
| Cranial Base | S-N | 0.527*** | 0.266* | 0.2225 | 0.8316*** | 0.4446 |
| Cranial Base | S-Ba | 0.3593** | 0.1393 | 0.0757 | 0.4041 | 0.2519 |
| Cranial Base | Ba-S-N | −0.2891* | 0.0578 | −0.0178 | −0.2507 | 0.0777 |
indicates p<0.05
indicates p<0.01
indicates p<0.001
Cephalometric measurements of patients with known sphenoid wing dysplasia and/or tibial dysplasia
We identified one 50-year-old male with NF1 and known left sphenoid wing dysplasia resulting in a large defect of his left orbit. He had multiple operations as a child to remedy this defect. He also had persistent non-union of fractures of his left ulna, requiring recurrent casting and bone grafting. A 67-year-old male with NF1 had left tibial dysplasia, fracture, non-union and pseudoarthrosis and required many years of casting and bone grafts. He did not have known sphenoid wing dysplasia. Supplemental Table 5 lists the 16 cephalometric measurements, height, head circumference, interpupillary distance measurements from these two patients.
Discussion
In our study of 101 persons with NF1 (78 adults and 23 children) with age-, ethnicity- and gender-matched controls, we found a shorter maxilla, mandible, cranial base, (especially anteriorly) and diminished facial height in adults, but not children, with NF1. The cranial base angle was significantly more oblique in adults with NF1. Some measures of facial height, mandible size and anterior cranial base correlated with height, less so with head circumference and not at all with interpupillary distance. When stratified by tall and short stature (defined post hoc by ≥ or < 20th centile height of the general population), AFH, LAFH and Co-Gn (mandible length) were significantly smaller in the shorter adults; anterior cranial base length (S-N) was of borderline significance. However among taller NF1 patients, whose height may be more similar to that of the reference population, there was no significant difference in any measurement of facial height. Curiously, the angle Ba-S-N, describing the sagittal relationship of the mandible to the cranial base, became more distinctly oblique, and the angle ANB, denoting the sagittal relationship between maxilla and mandible, became more distinctly acute. These results suggest that some differences in NF1 (e.g., facial height, length of maxilla) may due to differences in height while others (e.g., ANB, Ba-S-N) may be due to NF1 independent of height. In this dataset of American whites with NF1, we confirmed (in a modestly larger sample) many of the findings found by Heerva et al. [8] in an NF1 Finnish cohort.
Cephalometry is an inexpensive, readily available and non-invasive technique (albeit associated with ionizing radiation exposure) that historically has been under-utilized in studying the cranio-facial phenotype in NF1. We wondered what objective insight into the NF1 facies, said to feature hypertelorism and a broad nasal base [14], cephalometric analysis could provide. We hypothesized that cephalometry may also be able to provide some clues to the pathogenesis of sphenoid wing dysplasia, a rare complication of NF1.
Bony abnormalities in NF1 are well-known and are traditionally categorized as either generalized (osteopenia/osteoporosis, shortness of stature) or focal (tibial dysplasia, scoliosis, sphenoid wing dysplasia). Sphenoid wing dysplasia is estimated to affect up to 11% of persons with NF1 and has traditionally been thought to arise secondary to an adjacent soft tissue abnormality (e.g., plexiform neurofibroma) [5–7]. Another hypothesized explanation for the finding is a defective bone cell-autonomous program [5]. Two lines of evidence support this second possibility. First, NF1 sphenoid wing lesions are significantly more likely to be associated with other dysplasias of the tibia and vertebrae [15] and, second, the formation of the sphenoid bone (summarized below) arises from endochondral bone formation, which is known to be abnormal in NF1 [3].
The sphenoid bone is a major component of the anterior cranial base. The entire cranial base is a midline structure composed of basioccipital, sphenoid, ethmoid, and frontal bones in the midline, and temporal bones laterally. The early embryologic precursor of the cranial base is a cartilaginous plate, later replaced by bone through endochondral ossification [16]. Individual bones are then connected by cartilaginous structures, called synchondroses, which are morphologically similar to long bone growth plates [17, 18]. The anterior and posterior portions of the cranial base, demarcated by the sella turcica, develop at different rates. The linear growth of the anterior cranial base is approximately twice that of the posterior cranial base [16, 19], due to its earlier ossification and maturation. The cribriform plate of the ethmoid bone, which constitutes a significant portion of the anterior cranial base, completes its growth at the end of the second year postnatally [17]. Thereafter, growth of the remainder of the anterior cranial base occurs at the sphenoethmoidal synchondrosis and the cartilage between mesethmoid and frontal bones as long as the cartilage persists.
Our data showed that both the anterior and posterior cranial bases in NF1 patients are shorter than those in age- and gender-matched controls. The mean difference in anterior cranial base length reduction (−4.8%) was slightly more marked than the posterior cranial base length reduction (−3.8%). This difference could be explained by the difference in growth between the anterior base and the posterior cranial base, as noted above [17]. Although both the anterior and posterior cranial bases ossify by endochondral ossification, cartilaginous growth, known to be abnormal in NF1 [3], has a greater influence on anterior cranial base growth, especially at the sphenoethmoidal synchondrosis. We speculate that this may be likely responsible for the greater difference in the anterior cranial base compared with the posterior cranial base in the NF1 group. Although the anterior cranial base grows more rapidly than the posterior cranial base, the cranial base cartilage as a whole is regarded as an autonomous growth unit in the skull. This is especially true of the spheno-occipital synchondrosis, which provides a “pressure-adapted” growth mechanism, where compression from the weight of the brain and face bear down on this midline fulcrum-like synchondrosis. This pressure on the spheno-occipital synchrondrosis results in elongation of the midline cranial base [20]. Another measure of this process is the cranial base angle, which is significantly more oblique in NF1. Given that the brain in individuals with NF1 is larger on average than that of controls [21], we would expect increased pressure on the spheno-occipital synchrondrosis and even greater elongation of the midline cranial base. However, the cranial base in NF1 is shorter and cranial base angle more oblique than in controls, despite the greater pressure, consistent with a fundamental derangement in the growth program of the synchondrosis or bony components of the cranial base, including the sphenoid bone.
The differences we observe in NF1 cranial base growth support the hypothesis that there is an intrinsic fault in the bone cell-autonomous program. The resulting derangements in the sphenoid bone (or adjacent structures), perhaps due to abnormalities in cartilaginous growth [3] may explain its predilection to dysplasia. Focal bony lesions like sphenoid wing dysplasia, almost always unilateral, might be hastened by a “second hit” event in the gene NF1, much like NF1-associated pseudoarthosis [22, 23]. In a series of 16 individuals with NF1-associated pseudoarthosis, biallelic, pathogenic variants in NF1 (or somatic loss of heterozygosity) was detected in 75% of tissues or cultured cells from surgically resected pseudoarthrosis pathologic material [24]. Second hit events in NF1 likely occur in all developing osseous structures in an individual with NF1; certain bones like the sphenoid appear especially vulnerable to this sort of event. However, to date, we note that no second hit in NF1 has been reported in any individual with sphenoid wing dysplasia. In the one adult in our cohort with known sphenoid wing dysplasia, we observed shorter anterior and posterior cranial bases and a remarkably more acute cranial base angle relative with other NF1 adult males (Supplemental Table 5), although how many of these changes are secondary to his multiple surgical interventions is unknown.
The cranial base also exerts great influence on facial growth and plays an important role in coordinating craniofacial growth. During its growth, it carries the upper-middle face forward, inferiorly and laterally. A defect in anterior cranial base growth is often accompanied by midfacial deficiency [16]. Our data showed that maxillary length is significantly shorter in NF1 patients. Thus, NF1 patients appear to have a mid-face deficiency due to a combination of a smaller maxilla and reduced projection due to the shortened anterior cranial base despite a normal SNA (sagittal relationship of maxilla to cranial base). The SNA was relatively unchanged because both structures are shortened.
The shortened cranial base may give rise to differences in facial projection and cranial shape [25]. Sphenoid bone size is critical in determining the degree of facial projection and the position of the back of the face relative to the cranial base in modern humans [25]. Reduced facial projection in modern humans compared to other Homo taxa (including Neanderthals) is secondary to sphenoid reduction and thus shortened anterior cranial base [25]. Since the anterior cranial base is even shorter in individuals with NF1 compared with controls, this predicts even greater reduction in facial projection in these patients, with implications for the NF1 facies. We observed a significantly more oblique cranial base angle (also known as basicranial flexure) which results in a deficient projection of the midface and mandible. Interestingly, in modern humans reduction in facial projection from sphenoid shortening results in increased overall cranial globularity [25]. This suggests that the macrocephaly observed in NF1 patients arises not only from overgrowth of the brain but also as a consequence of sphenoid reduction.
From a dysmorphic perspective, the cephalometric differences in individuals with NF1 suggests that, in adults, we would expect a shorter face, mid-face hypoplasia, reduced facial projection, smaller jaw, and increased braincase globularity. These differences are not nearly as pronounced in children in this study, who may still be growing. With long-term follow-up, we may be able to determine or predict cranial base and maxillary growth deficiencies. In contrast to earlier studies[14], calculation of IPD from inner and outer canthal distances showed that only 4.7% of adults with NF1 had IPD greater than the 95th centile, suggesting hypertelorism is not a feature of NF1. IPD may seem larger to observers due to the short stature and large head size, but the distance is not substantively increased compared to population norms. For most people, the cephalometric differences are modest and likely not clinically detectable. Taken together, this “NF1 facies” is likely driven by sphenoid shortening, inferred from reduction in cranial base length, which can be directly observed by cephalometry. Finally, we hypothesize that NF1 sphenoid shortening, a common event, is consistent with an intrinsic NF1 bone cell defect, which, in turn, renders the bone more vulnerable to a random “second hit” in NF1, leading to sphenoid wing dysplasia, a rare event.
Conclusions
In our study of 101 persons with NF1 (78 adults and 23 children) with age-, ethnicity- and gender-matched controls, we found a shorter maxilla, mandible, cranial base, (especially anteriorly) and diminished facial height in adults, but not children, with NF1. The cephalometric differences in adults arise in part from cranial base shortening and thus result in a shorter face, mid-face hypoplasia, reduced facial projection, smaller jaw, and increased braincase globularity. Hypertelorism was identified in only one adult with NF1. For most people, these differences are modest and likely not clinically detectable. This “NF1 facies” is likely driven by sphenoid shortening, inferred from reduction in cranial base length, which can be directly observed by cephalometry. In addition, we suggest that NF1 sphenoid bone shortening is consistent with an intrinsic NF1 bone cell defect, which renders the bone more vulnerable to a random “second hit” in NF1, leading to sphenoid wing dysplasia in rare patients.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Supplementary Material
Supplemental Table 1. Difference between matched cases and controls age 18+ yrs stratified by sex
Supplemental Table 2. Difference between matched cases and controls age < 18 yrs stratified by sex
Supplemental Table 3. Height, head circumference and interpupillary distance among cases with NF1
Supplemental Table 4. Difference between matched cases and controls age 18+ yrs stratified at the 20th centile of height
Supplemental Table 5 Cephalometric measurements from two NF1 adults with known tibial dysplasia or sphenoid wing dysplasia
Acknowledgments
This work was supported by the Division of Cancer Epidemiology and Genetics (DCEG) and the Center for Cancer Research of the National Cancer Institute’s Intramural Research Program. We thank Janice Lee DDS, MD (NIDCR) for helpful discussions.
Footnotes
Author contributions: WC, LF, NK , ER and DS analyzed data. PG, CB, AB, BW and DS evaluated patients. WC, LF, NK and DS wrote the paper.
All authors reviewed the final draft of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman JM. Evaluation and Managment. In: Friedman JM, Gutmann DH, MacCollin M, Riccardi V, editors. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Johns Hopkins Press; Baltimore: 1999. [Google Scholar]
- 2.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O'Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 3.Kolanczyk M, Kossler N, Kuhnisch J, Lavitas L, Stricker S, Wilkening U, Manjubala I, Fratzl P, Sporle R, Herrmann BG, Parada LF, Kornak U, Mundlos S. Multiple roles for neurofibromin in skeletal development and growth. Hum Mol Genet. 2007;16:874–886. doi: 10.1093/hmg/ddm032. [DOI] [PubMed] [Google Scholar]
- 4.Visnapuu V, Peltonen S, Tammisalo T, Peltonen J, Happonen RP. Radiographic findings in the jaws of patients with neurofibromatosis 1. J Oral Maxillofac Surg. 2012;70:1351–1357. doi: 10.1016/j.joms.2011.06.204. [DOI] [PubMed] [Google Scholar]
- 5.Elefteriou F, Kolanczyk M, Schindeler A, Viskochil DH, Hock JM, Schorry EK, Crawford AH, Friedman JM, Little D, Peltonen J, Carey JC, Feldman D, Yu X, Armstrong L, Birch P, Kendler DL, Mundlos S, Yang FC, Agiostratidou G, Hunter-Schaedle K, Stevenson DA. Skeletal abnormalities in neurofibromatosis type 1: approaches to therapeutic options. Am J Med Genet A. 2009;149A:2327–2338. doi: 10.1002/ajmg.a.33045. [DOI] [PubMed] [Google Scholar]
- 6.Jacquemin C, Bosley TM, Liu D, Svedberg H, Buhaliqa A. Reassessment of sphenoid dysplasia associated with neurofibromatosis type 1. AJNR American journal of neuroradiology. 2002;23:644–648. [PMC free article] [PubMed] [Google Scholar]
- 7.Jacquemin C, Bosley TM, Svedberg H. Orbit deformities in craniofacial neurofibromatosis type 1. AJNR American journal of neuroradiology. 2003;24:1678–1682. [PMC free article] [PubMed] [Google Scholar]
- 8.Heerva E, Peltonen S, Pirttiniemi P, Happonen RP, Visnapuu V, Peltonen J. Short mandible, maxilla and cranial base are common in patients with neurofibromatosis 1. European journal of oral sciences. 2011;119:121–127. doi: 10.1111/j.1600-0722.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 9.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 10.Clementi M, Milani S, Mammi I, Boni S, Monciotti C, Tenconi R. Neurofibromatosis type 1 growth charts. Am J Med Genet A. 1999;87:317–323. doi: 10.1002/(sici)1096-8628(19991203)87:4<317::aid-ajmg7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Bushby KM, Cole T, Matthews JN, Goodship JA. Centiles for adult head circumference. Arch Dis Child. 1992;67:1286–1287. doi: 10.1136/adc.67.10.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pryor HB. Objective measurement of interpupillary distance. Pediatrics. 1969;44:973–977. [PubMed] [Google Scholar]
- 13.Dodgson NA. In: Woods JO, Merritt SA, Benton SA, Bolas MT, editors. Variation and extrema of human interpupillary distance; SPIE Proceedings Vol 5291: Steroscopic Displays and Virtual Reality Systems XI; San Jose, CA USA: International Society for Optics and Photonics; 2004. [Google Scholar]
- 14.Westerhof W, Delleman JW, Wolters E, Dijkstra P. Neurofibromatosis and hypertelorism. Archives of dermatology. 1984;120:1579–1581. [PubMed] [Google Scholar]
- 15.Alwan S, Armstrong L, Joe H, Birch PH, Szudek J, Friedman JM. Associations of osseous abnormalities in Neurofibromatosis 1. Am J Med Genet A. 2007;143A:1326–1333. doi: 10.1002/ajmg.a.31754. [DOI] [PubMed] [Google Scholar]
- 16.Nie X. Cranial base in craniofacial development: developmental features, influence on facial growth, anomaly, and molecular basis. Acta odontologica Scandinavica. 2005;63:127–135. doi: 10.1080/00016350510019847. [DOI] [PubMed] [Google Scholar]
- 17.Ford EHR. Growth of the human cranial base. Am J Orthodontics. 1958;44:498–506. [Google Scholar]
- 18.Bassed RB, Briggs C, Drummer OH. Analysis of time of closure of the spheno-occipital synchondrosis using computed tomography. Forensic science international. 2010;200:161–164. doi: 10.1016/j.forsciint.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery N. A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology. 2002;44:358–366. doi: 10.1007/s00234-001-0753-z. [DOI] [PubMed] [Google Scholar]
- 20.Enlow DH. The Facial Growth Process. In: Enlow DH, editor. Facial Growth. W.B. Saunders Company; Philadelphia: 1990. p. 102. [Google Scholar]
- 21.Gutmann DH. Abnormalities of the Nervous System. In: Friedman JM, Gutmann DH, MacCollin MM, Riccardi VM, editors. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. The Johns Hopkins University Press; Baltimore: 1999. pp. 190–202. [Google Scholar]
- 22.Stevenson DA, Little D, Armstrong L, Crawford AH, Eastwood D, Friedman JM, Greggi T, Gutierrez G, Hunter-Schaedle K, Kendler DL, Kolanczyk M, Monsell F, Oetgen M, Richards BS, Schindeler A, Schorry EK, Wilkes D, Viskochil DH, Yang FC, Elefteriou F. Approaches to treating NF1 tibial pseudarthrosis: consensus from the Children's Tumor Foundation NF1 Bone Abnormalities Consortium. Journal of pediatric orthopedics. 2013;33:269–275. doi: 10.1097/BPO.0b013e31828121b8. [DOI] [PubMed] [Google Scholar]
- 23.Upadhyaya M, Huson SM, Davies M, Thomas N, Chuzhanova N, Giovannini S, Evans DG, Howard E, Kerr B, Griffiths S, Consoli C, Side L, Adams D, Pierpont M, Hachen R, Barnicoat A, Li H, Wallace P, Van Biervliet JP, Stevenson D, Viskochil D, Baralle D, Haan E, Riccardi V, Turnpenny P, Lazaro C, Messiaen L. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970–2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet. 2007;80:140–151. doi: 10.1086/510781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paria N, Cho TJ, Choi IH, Kamiya N, Kayembe K, Mao R, Margraf RL, Obermosser G, Oxendine I, Sant DW, Song MH, Stevenson DA, Viskochil DH, Wise CA, Kim HK, Rios JJ. Neurofibromin deficiency-associated transcriptional dysregulation suggests a novel therapy for tibial pseudoarthrosis in NF1. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29:2636–2642. doi: 10.1002/jbmr.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman DE. Sphenoid shortening and the evolution of modern human cranial shape. Nature. 1998;393:158–162. doi: 10.1038/30227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Difference between matched cases and controls age 18+ yrs stratified by sex
Supplemental Table 2. Difference between matched cases and controls age < 18 yrs stratified by sex
Supplemental Table 3. Height, head circumference and interpupillary distance among cases with NF1
Supplemental Table 4. Difference between matched cases and controls age 18+ yrs stratified at the 20th centile of height
Supplemental Table 5 Cephalometric measurements from two NF1 adults with known tibial dysplasia or sphenoid wing dysplasia