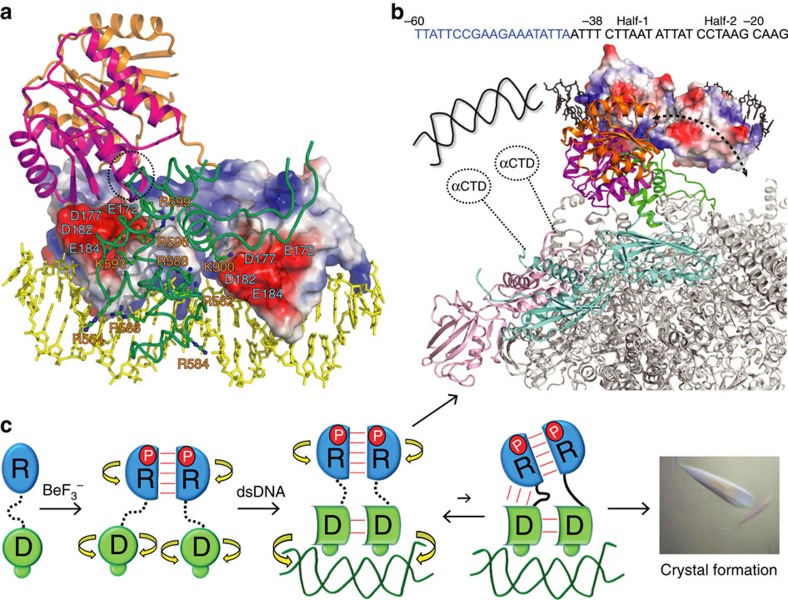
Figure 8. REC–DBD interdomain dynamics of PmrA and its possible role in RNAPH interaction.
(a) The docking model of PmrA–DNA–RNAPH complex with the REC of PmrA-1 is in magenta, PmrA-2 in orange and DNA in yellow. Surface charge distributions of two DBDs show the acidic patches. The basic patches on σ4 and β-flap tip helix of RNAPH (green) are shown in sticks. (b) Top view of the model with two α-subunits of RNAPH in pink and cyan and others in grey, showing that with stable REC–DBD interface, only the REC on PmrA-1 can interact with the RNAPH. However, if the REC dimer is connected to DBD with flexible linkers, the dimer can tumble freely and find the best orientation to interact extensively with RNAPH. In addition to these interactions, the C-terminal domain of the α-subunit of RNAPH may interact with the upstream DNA or with the PmrA–DNA complex. (c) A cartoon diagram summarizes the structure and dynamics of PmrA in transcription regulation. The REC is in blue and DBD in green. The phosphoryl analogue BeF3− is denoted as P. Linkers with high flexibility are shown as dotted lines and low mobility as solid lines. The interactions between domains are shown as red lines and the domains that can rotate independently by yellow curved arrows.