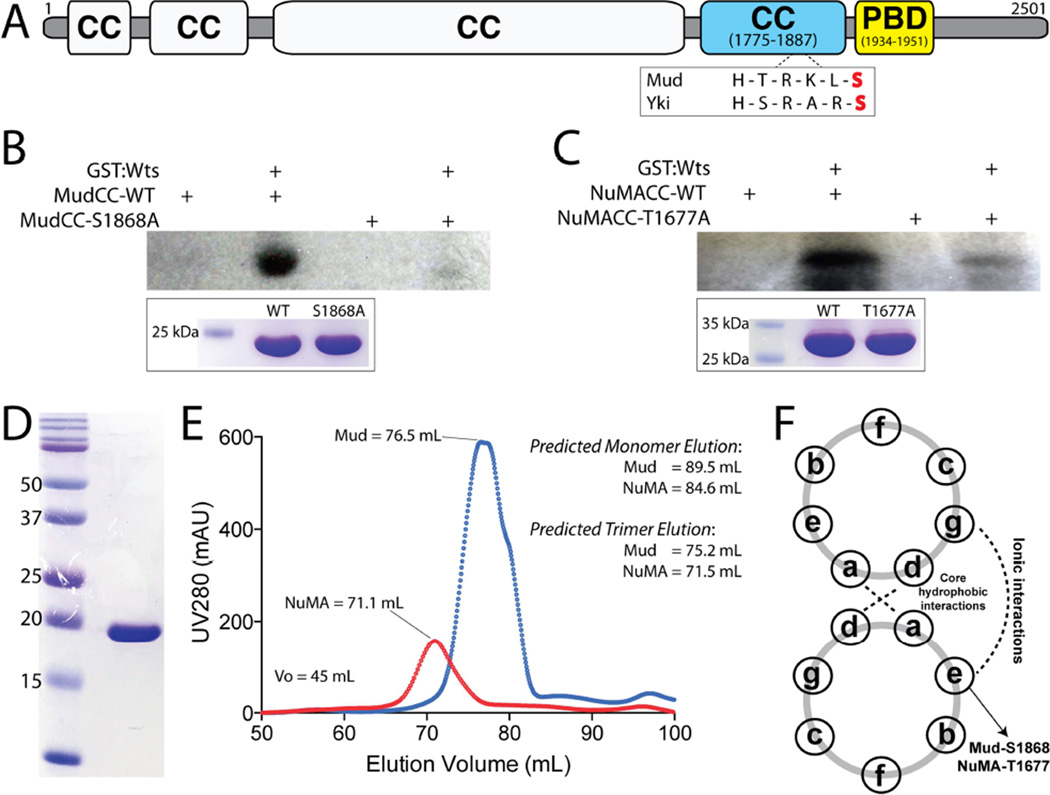
Figure 4. Warts directly phosphorylates Mud within its C-terminal coiled-coil domain.
(A) The domain architecture of Mud consists primarily of coiled coil (CC) domains. The most C-terminal of these (MudCC; blue) contains a single consensus phosphorylation motif for Wts kinase that closely resembles that found in Yki. Immediately following this domain is the minimal Pins-binding domain (MudPBD; yellow). (B) MudCC, wild-type (MudCC-WT) or a single S1868A mutant (MudCC-S1868A), were incubated in the absence or presence of GST:Wts kinase together with [γ-32P]-ATP. Radioactive phosphate incorporation was assessed by autoradiography using Kodak BioMax-MS radioisotope film. Inset below: Coomassie stain of reaction inputs showing equal levels of Mud protein between phosphorylation conditions. (C) Identical experiments carried out with the NuMACC domain and the corresponding T1677A mutant. (D) Coomassie stained gel indicating homogenous purity of the MudCC protein purification. (E) Both MudCC and NuMACC domains elute from a size-exclusion column at volumes consistent with trimers. Predicted elution volumes were calculated from a standard curve performed on the HiLoad Superdex 200 column (GE Healthcare). (F) Schematic of coiled-coil heptad repeat residue interactions. Both S1868 in MudCC and T1677 in NuMACC are predicted to reside at ‘e’ positions, which participate in ionic interactions in an ideal coiled-coil. See also Figure S2.