Figure 5.
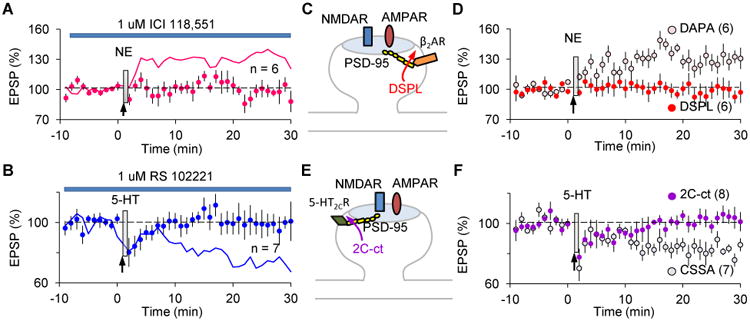
Anchoring of monoamine receptors is crucial for the transformation of transient LTP/D eligibility traces.
(A) The β2AR-specific antagonist ICI 118,551 (1 μM) prevents the transformation of the LTP eligibility trace by NE (95.2 ± 5.3%). The magenta line depicts control LTP (data from Fig1D).
(B) The 5-HT2CR specific antagonist RS 102221 (1 μM) prevents the transformation of the LTD eligibility trace by 5-HT (99.8 ± 8.2%). The blue line depicts control LTD (data from Fig1E). (C) β2AR directly interacts with PSD-95, and its c-terminal peptide DSPL disrupts this interaction.
(D) DSPL, but not the scrambled peptide DAPA, abolished the NE-mediated transformation of the LTP eligibility trace (DSPL: 96.1 ± 8.2%; DAPA: 127.8 ± 7.9%).
(E) The C-terminal peptide 2C-ct prevents the interaction between 5-HT2CR and PDZ-containing proteins such as PSD-95.
(F) 2C-ct, but not the control peptide CSSA, blocked transformation of the LTD eligibility trace by 5-HT (2C-ct: 102.9 ± 3.7%; CSSA: 82.6 ± 3.9%).
See also Fig S3-4.
