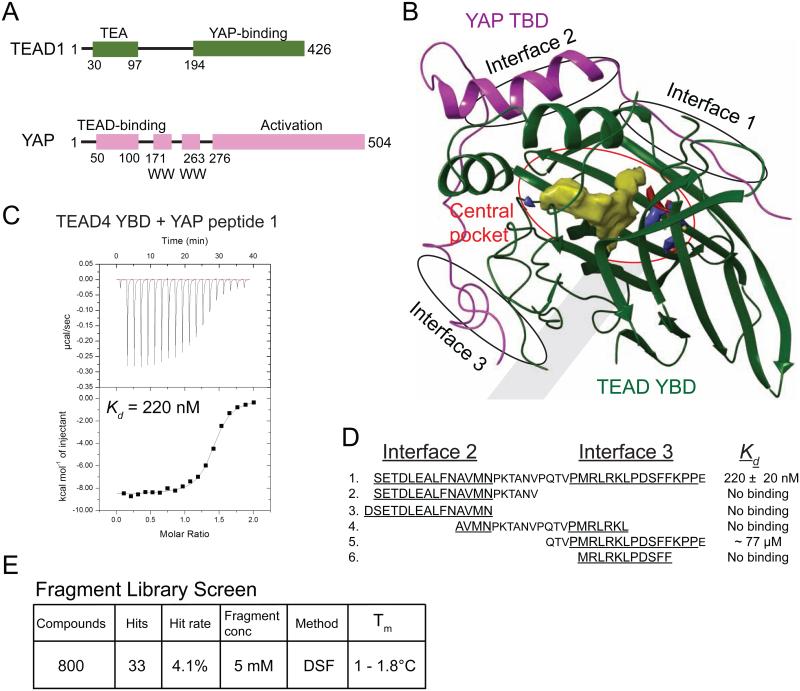
Figure 1. The YAP-binding Domain of TEADs Has a Central Pocket.
(A) Domain architecture of TEAD1 and YAP. All the four TEAD genes have a N-terminal DNA-binding TEA domain and a C-terminal YAP-binding domain (YBD). YAP has an N-terminal TEAD-binding domain (TBD) followed by one or two WW domains and a C-terminal activation domain.
(B) The crystal structure of the TEAD-YAP complex (PDB code 3KYS) reveals three interfaces between TEAD and YAP. TEAD YBD is colored green and YAP TBD is colored magenta. TEAD YBD has a large pocket in the center (red circle); the hydrophobic volume of this pocket is shown in yellow. On both ends of this pocket are hydrophilic areas, shown in red and blue that could be used to improve the specificity of TEAD-binding drugs. All structural figures were generated with Schrödinger software suite or Chimera (UCSF).
(C) Isothermal titration calorimetry (ITC) showing heat response during TEAD-YAP interaction. The binding affinity (Kd) between the YAP peptide 1 and TEAD was indicated.
(D) Sequences and binding affinities of the YAP peptides used in this study. Residues from Interface 2 and 3 are underlined.
(E) Summary of the results from fragment library screen.