Table 2.
Coupling of CO2 and various epoxides promoted by complexes 1 and 2.a
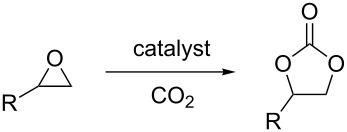 | ||||
| Entry | Catalyst | R | Conversionb (%) | Yieldc (%) |
| 1 | 1 | Ph | 100 | 62 |
| 2 | 1 | CH2OPh | 100 | 78 |
| 3 | 1 | p-ClPh | 100 | 95 |
| 4 | 1 | Bu | 100 | 80 |
| 5 | 1 | Et | 100 | 78 |
| 6 | 1 | Me | 100 | 52 |
| 7 | 1 | CH2Cl | 100 | 82 |
| 8 | 1 | CH2OH | 100 | 85 |
| 9 | 2 | Ph | 100 | 80 |
| 10 | 2 | CH2OPh | 64 | 56 |
| 11 | 2 | p-ClPh | 99 | 84 |
| 12 | 2 | Bu | 100 | 60 |
| 13 | 2 | Et | 100 | 71 |
| 14 | 2 | Me | 100 | 88 |
| 15 | 2 | CH2Cl | 100 | 80 |
| 16 | 2 | CH2OH | 100 | 76 |
aReaction conditions for catalyst 1: solvent free, 10 bar pressure of CO2, 35 °C, 24 h; for catalyst 2: solvent free, 10 bar pressure of CO2, 25 °C, 24 h. bDetermined by 1H NMR spectroscopy of the unpurified product. cAfter purification by column chromatography.
