Table 5.
RCM and ene–yne reactions catalysed by 93a–f and 94 in air.a
| Substrate | Productb | Catalyst (mol %) | T (°C)c | t (h) | Yield (%)d |
 29 |
 30 |
85 (1) 93a (1) 93b (1) 93c (1) 93d (1) 93e (1) 93f (1) 94 (1) |
30 30 30 30 30 30 30 30 |
0.4 1.7 1.7 1.7 1.8 1.7 1.9 0.9 |
42 96 93 7 92 97 17 90 |
 77 |
 78 |
85 (1) 93a (1) 93b (1) 93d (1) 93e (1) |
30 30 30 30 30 |
1.7 1.7 1.5 1.7 1.5 |
23 87 72 72 86 |
 95 |
 96 |
85 (5) 93a (5) 93b (5) 93d (5) 93e (5) |
30 30 30 30 30 |
0.4 0.4 0.4 0.4 0.4 |
40 41 38 36 35 |
 55 |
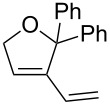 56 |
85 (2) 93a (2) 93b (2) |
30 30 30 |
6 5 8 |
94e 96e 96e |
aReaction conditions: Catalyst (mol %), nondegassed DCM (commercial-grade HPLC) (0.1 M) in air. bE = COOEt. cReactions at 50 °C were performed in nondegassed toluene (commercial-grade HPLC) in air. dYields determined by 1H NMR. eIsolated yields after flash chromatography [83].
