Abstract
Twenty-five years ago marked the publication of the first report describing a functional contribution by the cytokine, macrophage migration inhibitory factor (MIF), to tumor-associated angiogenesis and growth. Since first appearing, this report has been cited 304 times (as of this writing), underscoring not only the importance of this landmark study but also the importance of MIF in tumor neovascularization. Perhaps more importantly, this first link between MIF and stromal cell–dependent tumor angiogenesis presaged the subsequent identification of MIF in mediating protumorigenic contributions to several solid tumor stromal cell types, including monocytes, macrophages, T lymphocytes, NK cells, fibroblasts, endothelial progenitors and mesenchymal stem cells. This retrospective review will broadly evaluate both past and present literature stemming from this initial publication, with an emphasis on cellular sources, cellular effectors, signal transduction mechanisms and the clinical importance of MIF-dependent tumor vascularization.
HISTORICAL PERSPECTIVE
In the mid-1990s, studies led by Richard Bucala demonstrated that migration inhibitory factor (MIF) was a proinflammatory cytokine produced by macrophages that was not only required to mount innate immune responses but also was a major mediator of septic shock (1). A postdoctoral fellow in Bucala’s laboratory, Michael Bacher, had surprisingly discovered that MIF also was abundantly produced by T cells and was required for their activation and proliferation (2). During the course of his studies, he examined the relative secretion of MIF by primary and transformed macrophages and T cells and, in unpublished studies, observed that transformed hematopoietic cells secrete markedly elevated MIF relative to their nontransformed counterparts. The dual observations that MIF supported T-cell proliferation and was abundantly secreted by transformed cells led us to hypothesize that MIF may serve as an autotrophic growth factor for cancer cells.
A potent monoclonal antibody specific for MIF was found to be effective in preventing mortality from LPS-induced sepsis in mice and, given the translational goals of our laboratory, we initially elected to investigate the efficacy of this antibody in the 38C13 mouse B lymphoma model in vivo (before directly testing our hypothesis in vitro). We observed a marked reduction in B lymphoma growth in vivo and assumed that this was due to the effects of MIF inhibition on the proliferation of the B lymphoma cells (as had been established for T-cell proliferation). However, when we analyzed the B-cell lymphoma cells for MIF expression, we found that they did not express significant levels of MIF and, furthermore, the anti-MIF antibody had no effect on B-cell lymphoma proliferation (3). Perplexed, we examined the tumors after staining with hematoxylin and eosin and noticed a marked reduction in the clusters of residual red blood cells present in the vasculature after anti-MIF treatment. This result led us to stain the tumors for microvascular endothelial cells by using anti-CD31, which revealed a significant reduction in vascular density after anti-MIF administration. We then examined human endothelial cells for MIF secretion and for sensitivity to anti-MIF and discovered that the endothelial cells, unlike the B-cell lymphoma cells, were in fact secreting high levels of MIF, which in turn was required for their proliferation. On the basis of these studies, we concluded that the antitumor effects caused by MIF inhibition were due in part to the effects of MIF inhibition on angiogenesis (3).
Importantly, this study was the first demonstration that MIF may be a valid target for the development of anticancer agents. Today, a phase I trial of an anti-MIF antibody is underway in patients with advanced solid malignancies. Louis Pasteur famously stated that “chance favors the prepared mind,” but the truth is that we were simply lucky to observe the reduction in vasculature caused by the anti-MIF antibody.
CELL SOURCES OF MIF-DEPENDENT TUMOR ANGIOGENESIS
As described above, our study suggested that the primary source of soluble, extracellular MIF in 38C13 subcutaneous tumors was not from the tumor cells themselves but rather from endothelial cells in CD31+ microvessels within tumor stroma (3). This result was somewhat surprising given the recent descriptions at the time that MIF is overexpressed in primary and metastatic human (4) and murine (5) tumors and tumor cell lines. In contrast, our study found no evidence of MIF expression or functional activity in the 38C13 B-cell lymphoma cell line. The apparently unintended use of a very low to null MIF-expressing cell line for those studies turned out to be fortuitous, since this allowed for the discovery of functional MIF expression in tumor-associated endothelium (3). Because anti-MIF treatment effectively inhibited microvascular endothelial cell (EC) proliferation and Matrigel-implanted neovascularization, we concluded that autocrine-acting EC-derived MIF is necessary for maximal tumor-associated angiogenesis.
Several published (5–11) and unpublished (Mitchell laboratory) studies find that MIF is highly expressed in the vast majority of human and mouse solid tumors and tumor cell lines. In a study that was published shortly after our study, Shimizu et al. (10) reported that MIF is expressed in human melanoma cell lines and anti-MIF reduces xenografted melanoma-associated angiogenesis in a dorsal air sac implant study. Unlike our prior finding demonstrating that EC-derived MIF is necessary for 38C13 tumor outgrowth, the implication from the Shimizu study was that tumor-derived MIF was acting in a paracrine fashion to promote microvessel maturation. Although it is possible that the anti-human MIF used in the Shimizu study sufficiently neutralized the mouse EC-derived MIF, thus accounting for the observed phenotypes, it is more likely that paracrine-acting, tumor-derived MIF was in fact responsible for promoting the observed angiogenic phenotypes (10).
Further complicating the issue and reminding us that the macrophage was the first effector cell type described for MIF-dependent activities (12,13), White et al. (14) reported that human non–small-cell lung carcinoma (NSCLC)-derived MIF activates cocultured macrophages to express and secrete angiogenic chemokines such as interleukin (IL)-8, ENA-78 and chemokine (C-X-C motif) ligand 1 (CXCL-1). This study was notable for three independent reasons. First, it was the first description of monocyte/macrophage cell populations being demonstrated as targets of MIF-mediated angiogenesis. Second, and perhaps more importantly, it foretold of the subsequent identification of MIF as a central determinant of tumor-associated macrophage (TAM) alternative activation (15). Third, this was the first description of MIF being over-expressed and functionally active in human NSCLC cells, a tumor type that is now well documented to require MIF for a number of tumor-promoting processes (16–20).
One of the most profound examples of MIF-dependent neovascularization stems from the finding that bone marrow– derived macrophages (BMDMs) recruited to the stroma of teratomas express and secrete MIF that, in turn, dictates the vast bulk of microvessel density within tumor lesions (21). In this study, syngeneic embryonic stem cells injected into MIF-deficient mice grew tumors that were a fraction of the size of those developing in MIF wild-type mice, a finding that was attributed to profoundly defective teratoma vascularization. Importantly, reconstitution of MIF wild-type bone marrow into MIF-deficient mice restored microvessel density within the teratomas and, accordingly, restored tumor burden (21). Perhaps even more strikingly, MIF-deficient bone marrow reconstitution dramatically reduced tumor burden in adoptively transferred MIF wild-type mice. Phenotypes associated with bone marrow reconstitution were found not to be due to endothelial cell progenitors that populate bone marrow but rather due to BMDMs. Specifically, mobilized BMDMs that infiltrated developing teratomas were shown to express/secrete high levels of MIF that, in turn, directly promote pericyte differentiation and endothelial cell proliferation (21).
The BMDM study findings were in line with results from a study designed to directly evaluate the relative contributions of tumor-derived MIF versus stromal-derived MIF in mouse models of melanoma (22). In this study, B16-F10 melanoma cells were rendered MIF-deficient by using small hairpin RNA (shRNA), and MIF-competent and MIF-deficient B16-F10 cells were implanted subcutaneously into MIF+/+ and MIF−/− mice. Interestingly, tumor-derived MIF was found to be almost entirely dispensable for tumor outgrowth in MIF+/+ mice, whereas the growth of MIF-containing and MIF-deficient B16-F10 cells were equally retarded when implanted into MIF−/− mice. Reductions in the size of implantable melanoma lesions in MIF−/− mice correlated with substantial reductions in microvessel density within the tumor lesions of these mice. These findings suggested a critical role for stromal-derived, but not tumor-derived, MIF in B16-F10 melanoma-induced angiogenesis and accompanying tumor outgrowth (22).
Combined, results from these studies indicate that the cellular sources of MIF may be distinctly different depending on the tumor type, and perhaps the location, of the malignant lesion. Endothelial cells, tumor cells and tumor-infiltrating macrophages can independently produce, respond to, and secrete copious amounts of extracellular MIF that, in turn, can promote tumor neovascularization in a variety of ways.
CELL EFFECTORS OF MIF-DEPENDENT TUMOR ANGIOGENESIS
Regardless of which cells are responsible for MIF release into tumor microenvironments, early studies clearly indicated a role for MIF as an endothelial cell-activating proangiogenic cytokine that acts directly on ECs to enhance proliferation (3), motility (14) and, ultimately, neovascularization. Although this paradigm is undoubtedly true and functional within most, if not all, malignant lesions, MIF-dependent tumor angiogenesis is made up of a number of other indirect factors that, cumulatively, serve to coordinate tumor-associated neovascularization. One example discussed above is that of tumor-derived MIF-activating stromal macrophages, in a paracrine fashion, to secrete additional angiogenic cytokines and growth factors that, in turn, can efficiently and directly recruit and activate tumor ECs (14). In this case, macrophages activated by paracrine-acting, tumor-derived MIF significantly upregulate the expression and secretion of angiogenic CXC chemokines that can in turn activate ECs independently of MIF. This illustrates yet another mode of action by which tumor stroma–associated MIF can promote neovascularization in developing neoplasms.
As discussed above, MIF can promote effector cell activation as both an autocrine-acting and a paracrine-acting cytokine. In another example of autocrine-dependent neoangiogenic promotion, MIF overexpressed in malignant human NSCLC cell lines was found to be necessary for the maximal expression and secretion of vascular-endothelial cell growth factor (VEGF) and IL-8 (17). Notably, this was the first description of a redundant, additive function for the only known homolog of MIF, D-dopachrome tautomerase. Individual siRNA targeted knockdown of either MIF or D-dopachrome tautomerase (D-DT) in human NSCLC cells reduces NSCLC cell– derived VEGF and IL-8 expression by ~30%, but simultaneous knockdown reduces angiogenic growth factor expression and resultant NSCLC supernatant-induced human umbilical vein endothelial cell tube formation by 70–80%. Importantly, MIF and D-DT were equally efficient at rescuing the siRNA phenotypes in add-back studies, indicating a shared mode of action. It should be noted that a subsequent study by Tarnowski et al. (23) also described a central requirement for MIF in dictating the expression of IL-8 and VEGF expression in human rhabdomyosarcoma cell lines.
An additional example of autocrine-acting, MIF-dependent, angiogenic growth factor production is that of monocyte/macrophage-derived MIF. Unlike tumor-derived MIF acting as an autocrine-acting factor (as described above) or paracrine MIF activating tumor stromal macrophages (14), monocyte/macrophage-derived MIF was recently discovered to be a critical determinant of TAM angiogenic growth factor production (15). By using models of primary and metastatic melanoma tumor growth in syngeneic mice, Yaddanapudi et al. (15) discovered that TAMs purified from MIF−/− mice exhibit profound defects in angiogenic growth factor expression compared with TAMs purified from MIF+/+ mice. Interestingly, this result occurred even though both B16-F1– and B16-F10–implanted tumor cells express copious amounts of both intracellular and secreted MIF. Additional evidence to support a conclusion that monocyte/macrophage-derived MIF is responsible for the defective production of angiogenic growth factors and chemokines in these cells came from the use of a small molecule inhibitor of MIF, 4-iodo-6-phenylpyrimidine (4-IPP) (15,18). Addition of 4-IPP to MIF+/+ TAMs purified from B16-F1 tumors results in a dramatic re-polarization of M2 (alternatively activated) to M1 (classically activated) TAM phenotype, which effectively phenocopies MIF−/− TAMs. These TAMs exhibit profoundly defective angiogenic growth factor expression (15).
The studies described above indicating a dominant role for macrophage-derived MIF in dictating TAM angiogenic potential independent of tumor-derived MIF brings up an interesting and as yet unresolved question regarding how autocrine/paracrine activities of this cytokine exert their respective phenotypes. Two independent studies (both evaluating B16 melanoma cell lines implanted into syngeneic mice) describe a tumor-independent MIF-mediated stromal contribution to melanoma angiogenesis and tumor progression (15,22). The primary question raised by these studies is why MIF, secreted at high levels into the extracellular space by tumor cells, does not rescue loss of stromal MIF. Assuming that loss of extracellular MIF is, in fact, responsible for the phenotypes observed with MIF stromal deficiency (15,22), there are at least two possible explanations that could explain how this might occur, although neither has been rigorously tested as of yet. The first possibility is that MIF secretion is coupled with the trafficking of a highly dynamic MIF receptor/receptor complex that is sensitive to time- and/or concentration-dependent ligand activation. In this scenario, MIF secretion may be coupled to CD74 receptor membrane localization, where the receptor is only transiently expressed on the cell surface before being internalized. If MIF is not cosecreted during CD74 membrane localization, efficient receptor ligation may not occur and unoccupied CD74 could be reinternalized without an accompanying MIF-activation signal (24,25).
Another possible explanation could lie in differential processing and/or posttranslational modification of MIF in tumor cells versus stromal monocytes/macrophages. In this scenario, tumor-derived secreted MIF could be modified or could be lacking a modification that does not allow for the subsequent paracrine activation of receptors/receptor complexes on stromal cells. Because MIF has been found to be post-translationally modified in a manner that is suggested to influence its extracellular activation properties (26–28), it is certainly feasible that this may be a possible explanation for why tumor-derived MIF cannot functionally compensate for loss of stromal MIF. Regardless of the underlying mechanism, it is clear that much more study is needed to delineate the differential regulation of this important signaling paradigm that exists between tumor- and stromal-derived MIF.
SIGNALING DETERMINANTS OF MIF-DEPENDENT TUMOR ANGIOGENESIS
Although MIF was one of the first cytokine activities ever described (12,13), a cell surface receptor for MIF was not identified until nearly 40 years after the initial discovery of MIF (29). Since its identification (29), the type I cell surface receptor, CD74, has been repeatedly validated as being a central determinant of outside-in signaling initiated by MIF (30). CD74 activation has been linked to MIF and D-DT– dependent autocrine regulation of VEGF and IL-8 transcription in human lung adenocarcinoma cells (17). In this study, MIF and/or D-DT–mediated VEGF and IL-8 expression in NSCLC cells required CD74-dependent c-Jun N-terminal kinase/stress-activated protein kinase activation and subsequent c-Jun/AP-1–dependent transcription (17).
In a separate study evaluating autocrine MIF-dependent proangiogenic growth factor expression in rhabdomyosarcoma cell lines, MIF was found to similarly support VEGF and IL-8 expression but did so via the activation of CXCR2, CXCR4 and/or CXCR7 chemokine receptor(s) (23). Interestingly, while several reports indicate that non-canonical MIF-mediated chemokine receptor activation requires the functional interaction of CXCR2 and/or CXCR4 with MIF’s primary cell surface receptor CD74 (31–33), rhabdomyosarcoma cells were found to not express CD74 (23). This result suggests that outside-in signaling by MIF (and likely D-DT) can proceed using a number of different CD74, CXCR2, CXR4 and/or CXCR7 receptor/coreceptor combinations. This receptor promiscuity in initiating signal transduction pathways may provide some insight into the highly pleiotropic nature of MIF and its physiologic and pathophysiologic activities (34), especially those associated with neovascularization (35).
Stabilization of hypoxia-inducible factor-alpha (HIF-α) transcription factors by low oxygen tension in developing tumors is arguably the most important determinant of solid tumor neovascularization (36). MIF was first linked to tumor hypoxic responses when it was reported that MIF mRNA levels are induced by low oxygen tensions in human squamous carcinoma cell lines (37). Although subsequent studies confirmed these findings (38–40), it was not until a report by Baugh et al. (41) that MIF was confirmed as a direct target of HIF-mediated transcription. The first description of hypoxia-induced MIF acting as a functional effector of HIF-dependent hypoxic adaptation came when Winner et al. (6) reported that hypoxia-induced VEGF expression is dramatically reduced in MIF-deficient cells. This defect in HIF-1α–dependent VEGF transcription was found to be due to an inherent requirement for MIF in hypoxia-induced HIF-1α protein stabilization creating, in essence, an amplification loop between hypoxia, MIF and HIF-1α (6). Consistent with these findings, MIF overexpression in human breast cancer cell lines was found to promote hypoxia-induced HIF-1α stabilization (42). Interestingly, this study revealed that CD74 is necessary for MIF-dependent HIF-1α stabilization.
TRANSLATIONAL/CLINICAL RELEVANCE OF MIF-DEPENDENT TUMOR ANGIOGENESIS
As discussed above, both normoxic and hypoxia-induced VEGF expression is significantly reduced in MIF-deficient cells and increased in MIF overexpressing malignant cells (6,17,23,42). Consistent with a requirement for MIF in dictating maximal tumor-derived VEGF expression, numerous reports demonstrate that MIF intratumoral expression correlates with VEGF expression, tumor vessel density and risk of recurrence after resection in a variety of human cancers (43–49). In mouse models, MIF-deficient mice crossed to adenomatous polyposis coli (ApcMin/+) oncomice are characterized by significant reductions in both the number and size of adenomas that correspond to diminished tumor microvessel density (50). Moreover, MIF-deficient mice show a 45% reduction in chronic ultraviolet B (UVB) irradiation–induced epidermal tumorigenesis (51). The decreased tumor incidence and delayed tumor outgrowth in these MIF-deficient mice exposed to UVB correlated with significantly less VEGF expression and intratumoral microvessel density (51). Thus, one of the most consistent phenotypes associated with loss or inhibition of MIF in tumorigenesis is decreased angiogenic growth factor expression leading to microvascular density.
In addition to the clinical relevance of MIF overexpression in malignant cells and its highly correlative relationship to tumoral microvascular density, there is also evidence to suggest that MIF-dependent modulation of TAMs provides equally, if not more, clinically relevant contributions to human metastatic cancer disease progression. In a study by the Dranoff group, late-stage melanoma and ovarian cancer patients showing durable objective responses to an experimental immunotherapeutic were found to have circulating autoantibodies that specifically neutralize MIF-dependent, proangiogenic TAM responses (52). Patients responsive to anti–cytotoxic T lymphocyte–associated protein 4 (anti–CTLA-4; ipilimumab) plus a granulocyte-macrophage colony-stimulating factor (GM-CSF) vaccine (GVAX) had high levels of naturally occurring, anti-MIF autoantibodies that functionally inhibited MIF-dependent Tie-2 and matrix metalloprotease-9 (MMP9) expression in human TAMs. Autoantibodies were proposed to trigger tumor vasculopathies characterized by disrupted tumoral vasculature, lymphocyte/granulocyte infiltrates and, by extension, a significantly improved prognosis (52). Combined with preclinical mouse models that essentially phenocopy these findings in late-stage melanoma patients (15,21), it is not unreasonable to conclude that MIF is critically and centrally involved in monocyte/macrophage-dependent solid tumor vascularization.
Given the abundance of preclinical and clinical evidence suggesting a dominant functional contribution by MIF to human solid tumor vascularization and subsequent disease progression, it is highly conceivable that therapeutic targeting of MIF (either alone or in combination) could provide significant clinical efficacy. Toward this goal, a phase I clinical trial testing a humanized anti-MIF antibody is currently being evaluated in patients with advanced malignant disease in a variety of cancers (ClinicalTrial.gov protocol NCT01765790). Additionally, small molecule inhibitors of MIF also demonstrate significant efficacy in disrupting tumor neovascularization and ensuing primary and metastatic disease progression in preclinical disease models (15,53).
CONCLUSIONS
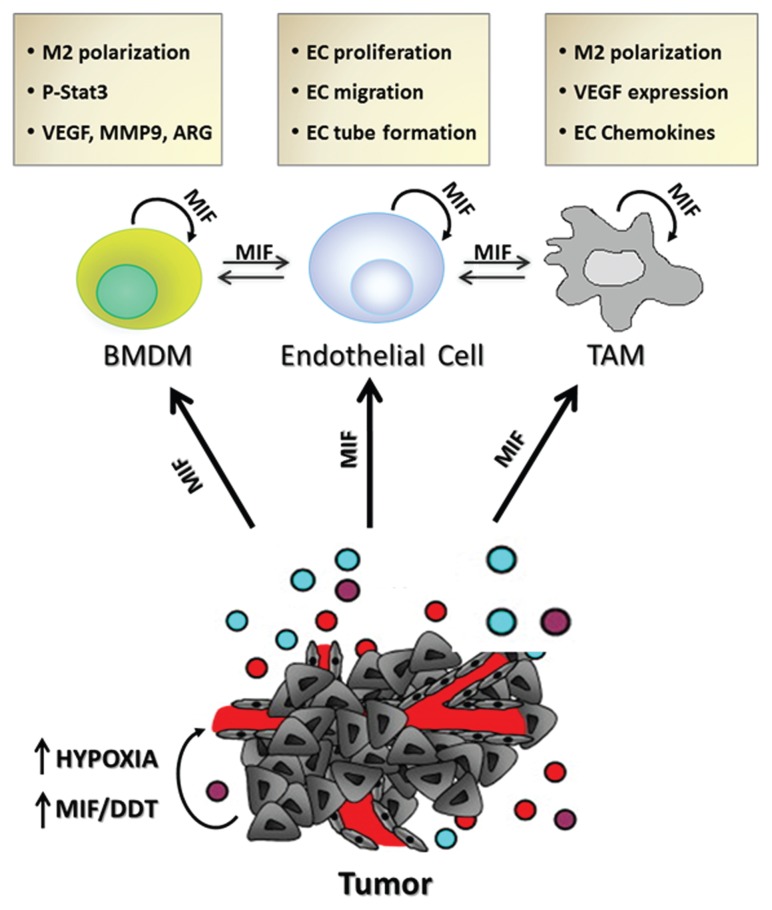
In the 25 years since the first report describing a functional role for MIF in tumor-associated angiogenesis was published in Molecular Medicine, literally hundreds of studies have validated and expanded on this initial observation. It is now abundantly evident that MIF secreted from numerous cell sources and acting, in both autocrine and paracrine manners, on numerous cell types is centrally important in promoting tumor-associated angiogenesis (Figure 1). Perhaps more importantly, MIF-dependent tumor neovascularization likely allows for primary and metastatic disease progression and ultimately contributes to increased patient mortality. It is hoped that the wealth of data that has been gathered and disseminated since this first report characterizing the contributions of MIF to angiogenesis will lead to new and improved chemotherapeutic targeting strategies designed to increase objective response rates and overall survival in patients with solid cancer malignancies.
Figure 1.
Schematic of autocrine and paracrine MIF angiogenic contributions to and from various cell effectors. ARG, arginase I; MMP9, matrix metalloprotease 9.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health grants NIH CA186661 and NIH CA102285 (both to RA Mitchell).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Chesney JA, Mitchell RA. (2015) 25 years on: a retrospective on migration inhibitory factor in tumor angiogenesis. 21 Suppl 1:S19–24.
REFERENCES
- 1.Calandra T, Bucala R. Macrophage migration inhibitory factor (MIF): a glucocorticoid counter-regulator within the immune system. Crit Rev Immunol. 1997;17:77–88. doi: 10.1615/critrevimmunol.v17.i1.30. [DOI] [PubMed] [Google Scholar]
- 2.Bacher M, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesney J, et al. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer-Siegler K, Hudson PB. Enhanced expression of macrophage migration inhibitory factor in prostatic adenocarcinoma metastases. Urology. 1996;48:448–52. doi: 10.1016/S0090-4295(96)00207-5. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi N, et al. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med. 1998;4:707–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 2007;67:186–93. doi: 10.1158/0008-5472.CAN-06-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer-Siegler K, Fattor RA, Hudson PB. Expression of macrophage migration inhibitory factor in the human prostate. Diagn Mol Pathol. 1998;7:44–50. doi: 10.1097/00019606-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Bando H, et al. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res. 2002;93:389–96. doi: 10.1111/j.1349-7006.2002.tb01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markert JM, et al. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu T, et al. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751–8. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- 11.Kamimura A, et al. Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer. 2000;89:334–41. [PubMed] [Google Scholar]
- 12.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966;56:72–7. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–2. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 14.White ES, Strom SR, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity. J Immunol. 2001;166:7549–55. doi: 10.4049/jimmunol.166.12.7549. [DOI] [PubMed] [Google Scholar]
- 15.Yaddanapudi K, et al. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J Immunol. 2013;190:2984–93. doi: 10.4049/jimmunol.1201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rendon BE, et al. Regulation of human lung adenocarcinoma cell migration and invasion by MIF. J Biol Chem. 2007;282:29910–8. doi: 10.1074/jbc.M704898200. [DOI] [PubMed] [Google Scholar]
- 17.Coleman AM, et al. Cooperative regulation of non-small cell lung carcinoma angiogenic potential by macrophage migration inhibitory factor and its homolog, D-dopachrome tautomerase. J Immunol. 2008;181:2330–7. doi: 10.4049/jimmunol.181.4.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winner M, et al. A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res. 2008;68:7253–7. doi: 10.1158/0008-5472.CAN-07-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brock SE, Rendon BE, Yaddanapudi K, Mitchell RA. Negative regulation of AMP-activated protein kinase (AMPK) activity by macrophage migration inhibitory factor (MIF) family members in non-small cell lung carcinomas. J Biol Chem. 2012;287:37917–25. doi: 10.1074/jbc.M112.378299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brock SE, Rendon BE, Xin D, Yaddanapudi K, Mitchell RA. MIF family members cooperatively inhibit p53 expression and activity. PLoS One. 2014;9:e99795. doi: 10.1371/journal.pone.0099795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, et al. MIF produced by bone marrow-derived macrophages contributes to teratoma progression after embryonic stem cell transplantation. Cancer Res. 2012;72:2867–78. doi: 10.1158/0008-5472.CAN-11-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard E, Strathdee C, Trueblood E, Queva C. Macrophage migration inhibitory factor produced by the tumour stroma but not by tumour cells regulates angiogenesis in the B16-F10 melanoma model. Br J Cancer. 2012;107:1498–505. doi: 10.1038/bjc.2012.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarnowski M, et al. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8:1328–43. doi: 10.1158/1541-7786.MCR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz V, et al. Role for CD74 and CXCR4 in clathrin-dependent endocytosis of the cytokine MIF. Eur J Cell Biol. 2012;91:435–49. doi: 10.1016/j.ejcb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Koch N, Moldenhauer G, Hofmann WJ, Moller P. Rapid intracellular pathway gives rise to cell surface expression of the MHC class II-associated invariant chain (CD74) J Immunol. 1991;147:2643–51. [PubMed] [Google Scholar]
- 26.Magi B, et al. Charge heterogeneity of macrophage migration inhibitory factor (MIF) in human liver and breast tissue. Electrophoresis. 1998;19:2010–3. doi: 10.1002/elps.1150191120. [DOI] [PubMed] [Google Scholar]
- 27.Watarai H, et al. Posttranslational modification of the glycosylation inhibiting factor (GIF) gene product generates bioactive GIF. Proc Natl Acad Sci U S A. 2000;97:13251–6. doi: 10.1073/pnas.230445397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim-Saijo M, Janssen EM, Sugie K. CD4 cell-secreted, posttranslationally modified cytokine GIF suppresses Th2 responses by inhibiting the initiation of IL-4 production. Proc Natl Acad Sci U S A. 2008;105:19402–7. doi: 10.1073/pnas.0810035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucala R, Shachar I. The integral role of CD74 in antigen presentation, MIF signal transduction, and B cell survival and homeostasis. Mini Rev Med Chem. 2014;14:1132–8. doi: 10.2174/1389557515666150203144111. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz V, et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009;583:2749–57. doi: 10.1016/j.febslet.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lue H, Dewor M, Leng L, Bucala R, Bernhagen J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell Signal. 2011;23:135–44. doi: 10.1016/j.cellsig.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klasen C, et al. MIF promotes B cell chemo-taxis through the receptors CXCR4 and CD74 and ZAP-70 signaling. J Immunol. 2014;192:5273–84. doi: 10.4049/jimmunol.1302209. [DOI] [PubMed] [Google Scholar]
- 34.Rex S, et al. The role of macrophage migration inhibitory factor in critical illness. Mini Rev Med Chem. 2014;14:1116–24. doi: 10.2174/1389557515666150203143736. [DOI] [PubMed] [Google Scholar]
- 35.Asare Y, Schmitt M, Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF): expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost. 2013;109:391–8. doi: 10.1160/TH12-11-0831. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Sun M, Wang L, Jiao B. HIFs, angiogenesis, and cancer. J Cell Biochem. 2013;114:967–74. doi: 10.1002/jcb.24438. [DOI] [PubMed] [Google Scholar]
- 37.Koong AC, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–7. [PubMed] [Google Scholar]
- 38.Takahashi M, et al. Macrophage migration inhibitory factor as a redox-sensitive cytokine in cardiac myocytes. Cardiovasc Res. 2001;52:438–45. doi: 10.1016/s0008-6363(01)00408-4. [DOI] [PubMed] [Google Scholar]
- 39.Schmeisser A, et al. The expression of macrophage migration inhibitory factor 1alpha (MIF 1alpha) in human atherosclerotic plaques is induced by different proatherogenic stimuli and associated with plaque instability. Atherosclerosis. 2005;178:83–94. doi: 10.1016/j.atherosclerosis.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 40.Bacher M, et al. Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol. 2003;162:11–7. doi: 10.1016/S0002-9440(10)63793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baugh JA, et al. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem Biophys Res Commun. 2006;347:895–903. doi: 10.1016/j.bbrc.2006.06.148. [DOI] [PubMed] [Google Scholar]
- 42.Oda S, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS One. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han I, et al. Expression of macrophage migration inhibitory factor relates to survival in high-grade osteosarcoma. Clin Orthop Relat Res. 2008;466:2107–13. doi: 10.1007/s11999-008-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagemann T, et al. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2007;261:147–57. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 46.Ren Y, et al. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann Surg. 2005;242:55–63. doi: 10.1097/01.sla.0000168555.97710.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shun CT, Lin JT, Huang SP, Lin MT, Wu MS. Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J Gastroenterol. 2005;11:3767–71. doi: 10.3748/wjg.v11.i24.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hira E, et al. Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer. 2005;103:588–98. doi: 10.1002/cncr.20818. [DOI] [PubMed] [Google Scholar]
- 49.White ES, et al. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–60. [PubMed] [Google Scholar]
- 50.Wilson JM, et al. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129:1485–503. doi: 10.1053/j.gastro.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 51.Martin J, et al. Macrophage migration inhibitory factor (MIF) plays a critical role in pathogenesis of ultraviolet-B (UVB)-induced nonmelanoma skin cancer (NMSC) FASEB J. 2008;23:720–30. doi: 10.1096/fj.08-119628. [DOI] [PubMed] [Google Scholar]
- 52.Schoenfeld J, et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 2010;70:10150–60. doi: 10.1158/0008-5472.CAN-10-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choudhary S, et al. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis. 2013;34:2891–9. doi: 10.1093/carcin/bgt239. [DOI] [PMC free article] [PubMed] [Google Scholar]