Abstract
Purpose
To evaluate longitudinal changes in circumpapillary retinal nerve fiber layer (RNFL) thickness, as measured by spectral-domain optical coherence tomography (SD-OCT), in children with optic pathway gliomas.
Design
Longitudinal cohort study
Methods
Global and quadrant specific circumpapillary RNFL thickness measures were acquired using either a hand-held during sedation or a table-top SD-OCT in children old enough to cooperate. Vision loss was defined as either a 0.2 logMAR decline in visual acuity, or progression of visual field. Percent change in circumpapillary RNFL thickness in eyes experiencing vision loss was compared to eyes with stable vision.
Results
Fifty-five eyes completed two-hundred fifty study visits. Ten eyes (18%) from 7 patients experienced a new episode of vision loss during the study and 45 (82%) eyes from 39 patients demonstrated stable vision across study visits. Percent decline of RNFL thickness between the baseline visit and first event of vision loss event was greatest in the superior (−14%) and inferior (−10%) quadrants as well as global average (−13%). Using a threshold of ≥ 10% decline in RNFL, the positive and negative predictive value for vision loss when two or more anatomic sectors were affected was 100% and 94%, respectively.
Conclusions
Children experiencing vision loss from their optic pathway gliomas frequently demonstrate a ≥ 10% decline of RNFL thickness in one or more anatomic sectors. Global average and the inferior quadrant demonstrated the best positive and negative predictive values. Circumpapillary RNFL is a surrogate marker of vision and could be helpful in making treatment decisions for children with optic pathway gliomas.
Spectral-domain optical coherence tomography (SD-OCT) measures of circumpapillary retinal nerve fiber layer (RNFL) thickness are used to diagnose and monitor a variety of genetic and acquired optic neuropathies.1–10 Understanding the relationship between functional decline (i.e., visual acuity or visual field loss) and structure changes (i.e., circumpapillary RNFL thickness) is essential to incorporating SD-OCT measures into patient management decisions. While glaucoma studies have provided the most insight about the structure function relationship, disease specific mechanisms undoubtedly impact this correlation.1,11–14 For example, most glaucoma patients experiencing visual field (VF) loss demonstrate relatively small changes in longitudinal measures of circumpapillary RNFL thickness (e.g., approximately 2 microns per year),15,16 although acute or under-treated glaucoma can produce more profound declines. On the other hand, patients with Leber’s hereditary optic neuropathy demonstrate a more rapid decline in retinal axons and vision.13,17–19
Children with optic pathway gliomas, low grade gliomas of the anterior visual pathway, require frequent ophthalmologic monitoring as they can experience visual acuity (VA) and or VF loss, typically progressing over a period of months to years.20 Despite treatment with chemotherapy and stability of tumor size, vision loss can occur multiple times throughout childhood.20–22 While quantitative VA testing is the recommended metric to monitor tumor progression, treatment response and guide management decisions in these young children,20,23,24 a surrogate marker of vision is desperately needed since most have difficulty cooperating and accurately completing quantitative VA and VF testing.20,23–25 Previous cross-sectional studies have demonstrated that the amount of circumpapillary RNFL loss is closely related to the magnitude of vision loss, and can readily discriminate between children with and without vision loss from their optic pathway gliomas.26,27 In order to determine if circumpapillary RNFL thickness can serve as a surrogate marker of vision in young children with optic pathway gliomas, obtaining SD-OCT measures before and after episodes of vision loss is necessary.
The purpose of this study was to examine longitudinal changes in circumpapillary RNFL thickness measures acquired before, during and after experiencing vision loss from an optic pathway glioma.
Methods
Patients
Children with optic pathway gliomas receiving their clinical care at Children’s National Medical Center, who were participating in a longitudinal study of SD-OCT between January 1, 2012 and December 31, 2014, were eligible for study enrollment. Written informed consent from the parent/guardian and written assent from the child when applicable was obtained before study enrollment. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Children’s National Medical Center Institutional Review Board. All data collected was HIPPA compliant.
Diagnosis of an optic pathway glioma was established by either biopsy demonstrating pathological features consistent with a WHO grade I or II low grade astrocytoma, or by radiographic features for those tumors isolated to the optic nerve. In children with neurofibromatosis type 1, optic pathway gliomas were diagnosed using established NIH criteria.28 Patients meeting all of the following inclusion criteria were enrolled: 1) a minimum of 3 separate SD-OCT imaging sessions that acquired technically acceptable circumpapillary RNFL measures; 2) successful quantitative VA testing at each study visit; and 3) no ophthalmologic or neurologic conditions, other than the optic pathway glioma, that could potentially damage their visual pathway and affect circumpapillary RNFL thickness measures (e.g., hydrocephalus, glaucoma). Patients experiencing vision loss within 3 months prior to study entry were excluded. Portions of data from previously reported studies were included if the additional study visits, meeting the above eligibility criteria, were obtained.29,30
Vision Testing
A complete ophthalmologic exam was performed at each study visit. Best corrected VA using quantitative testing methods (i.e., Teller grating acuity or standard recognition acuity methods) were chosen based on the child’s cognitive ability to perform the task using established protocols.25 VA loss was defined as a decline of 0.2 logarithm of the minimal angle of resolution (logMAR) or more compared to the baseline visit. Patients did not change VA testing formats during the study. Patient age and cooperation determined VF testing method and included testing by confrontation, automated perimetry using standard Humphrey 24-2 strategy (Carl Zeiss Meditec) or Goldmann kinetic perimetry. Using confrontation methods, only new VF defects determined to be reproducible were considered new events of VF loss. Progressive VF loss using Humphrey 24-2 was defined as three or more contiguous points reaching significance (P < 0.05). Humphrey VF were included if false-positive errors, false negative errors and fixation losses were less than 20%. Goldmann kinetic perimetry was tested using a minimum of V-4-E and I-4-E isopters. Extent of the VF along 12 vectors was confirmed by retesting the response. Goldmann kinetic perimetry VF loss was defined as any constriction greater than 10 degrees across a minimum of 3 contiguous 15 degree vectors using the V-4-E or I-4-E isopter. Either VA or VF loss could be considered an event of vision loss as both are indications for treatment. If VA and VF occurred at same study visit, this was considered to be one event of vision loss.
Clinical Characteristics
A standardized form was used to collect the following clinical characteristics; age, gender, race, ethnicity, diagnosis of Neurofibromatosis type 1, and location of optic pathway glioma on magnetic resonance imaging. Classification of optic pathway gliomas location were defined as 1) optic nerve only; 2) optic chiasm with or without optic nerve involvement; or 3) optic tracts with or without involvement of the optic nerves or chiasm. Patients with unilateral optic nerve gliomas could only contribute one study eye for the analysis as this was the only eye at risk for vision loss. Patients with abnormal vision at study entry could contribute two eyes to the analysis as they previously demonstrated the greatest inter-visit variability.29,30 Patients with normal vision who did not experience any events of vision loss could only contribute one study eye, chosen by a random number generator, as previous research from our laboratory has established inter-visit variability for circumpapillary RNFL thickness measures.29,30
SD-OCT Image Acquisition and Analysis
circumpapillary RNFL thickness measures were acquired using either a hand-held SD-OCT (Bioptigen, Research Triangle Park, NC) during sedation or a table-top SD-OCT (Spectralis, Heidelberg Engineering) in children old enough to cooperate. As stated in the inclusion criteria, at least one SD-OCT scan had to be acquired before the patient experienced a new episode of vision loss. All study visits were completed on the same SD-OCT device. Hand-held SD-OCT was acquired according to previously published protocols.26,30 After receiving mydriatric eye drops, hand-held SD-OCT imaging was performed approximately one hour later while the child was sedated for their clinically indicated magnetic resonance imaging to monitor their optic pathway glioma. Hand-held SD-OCT acquired a 6 × 6 × 2 mm volume scan centered over the optic nerve comprised of either 300 A-scans across 300 B-scans (2.5 second acquisition time) or 1,000 A-scans across 100 B-scans (2.8 second acquisition time) for all study visits. Alterations to the working distance of hand-held SD-OCT probe were made according to previously published recommendations and adjusted according to the child’s axial length and image quality.31 Scans with a quality index below 20 were excluded from analysis.32 Handheld OCT volumes were de-identified and analyzed by the same investigator (C-LC) using custom software with an established algorithm.33 If the software could not automatically detect the optic disc margin, it was drawn manually. Then a 3.45 mm circle was placed over the geometric center of optic nerve head and sampled along 1024 equally spaced A-scans. The 1024 A-scans were equally divided into 4 anatomic quadrants (superior, nasal, inferior, temporal) to measure the circumpapillary RNFL thickness.
In children old enough to cooperate, circumpapillary RNFL measures were acquired with the Spectralis SD-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) using the “Nsite Analytics” (version 5.6.3.0) and “TruTrack” eye tracking. The eye tracking feature allows the operator to “freeze” the infrared image and accurately center the 3.5mm circle over the optic nerve head. The highest image quality scan from the baseline visit was chosen as the reference scan, and all future acquisitions were acquired at the same location using the eye tracking feature. All scans were acquired in high-speed mode (768 A-scans) with an automatic real-time (ART) setting of 16. Scans with a signal strength < 20 db were discarded. circumpapillary RNFL thickness measures from the four anatomic quadrants (superior, nasal, inferior, temporal) and global average were recorded. All scans were reviewed for segmentation errors and image artifacts by the same investigator (CT-H).
Statistical Analysis
Demographic and clinical characteristics were summarized by standard descriptive statistics (e.g. means and standard deviations for continuous variables such as age and percentages for categorical variables such as gender). To account for differences between patient specific circumpapillary RNFL values at study entry, SD-OCT devices and SD-OCT segmentation algorithms, change in circumpapillary RNFL thickness was calculated as a percent change from baseline. A change of ≥10% was chosen a priori, based on previously published intra- and inter-visit reproducibility data.29,30 Wilcoxon rank-sum was used to compare baseline and final study visit thickness measures between those with stable vision and those who experienced vision loss during the study. Kaplan Meier curve and Cox proportional hazards models were used to assess the impact of SD-OCT measures and clinical characteristics on events of new vision loss. Data were analyzed using commercially available software (STATA, version 13; StataCorp, College Station, Texas).
Results
Fifty-five eyes from 46 patients met inclusion criteria and completed 250 study visits. Ten eyes (18%) from 7 patients experienced 18 distinct episodes of new vision loss during the study and 45 (82%) eyes from 39 patients demonstrated stable vision across a median of 4 study visits (Table 1). Patients were similar in age and most demographic features. A majority of those patients experiencing new vision loss had sporadic optic pathway gliomas whereas a majority of patients with stable vision had optic pathway gliomas secondary to Neurofibromatosis type 1. Ninety percent of patients experiencing new vision loss compared to 38% with stable vision were actively receiving treatment with chemotherapy and or biologic agents (i.e., bevacizumab, MEK inhibitor) during portions of the study. Three patients were excluded from the study since they could not cooperate with quantitative VA testing and another was excluded as they developed hydrocephalus that required treatment. None of the patients experienced a significant change (i.e,. ± 0.50 SE) in their refractive error during the study.
Table 1.
Demographic and Clinical Characteristics of Children with Optic Pathway Gliomas Experiencing New Onset Vision Loss or Stable Vision.
| New Vision Loss (N= 10) |
Stable Vision (N = 45) |
|
|---|---|---|
| Age, yrs (mean/median) | 6.9/5.8 | 6.5/5.5 |
| Range | (1.1 – 17.8) | (1.2 – 17.1) |
| Female sex, n (%) | 5 (50) | 30 (67) |
| Race, n (%) | ||
| White/Caucasian | 9 (90) | 34 (76) |
| Black/African American | 1 (10) | 6 (13) |
| Multiracial | 0 | 5 (11) |
| Ethnicity, n (%) | ||
| Non-Hispanic | 10 (100) | 42 (93) |
| Hispanic | 0 (0) | 3 (7) |
| Diagnosis, n (%) | ||
| NF1 – Optic Pathway Glioma | 2 (20) | 29 (64) |
| Sporadic – Optic Pathway Glioma | 8 (80) | 16 (36) |
| Treatment of Optic Pathway Glioma, n (%) | ||
| Never | 1 (10) | 18 (40) |
| During Study | 9 (90) | 17 (38) |
| Past | 0 (0) | 10 (22) |
| Total Visits, n (mean/median) | 67 (6.2/5) | 183 (4.0/4) |
| Range | (3 – 12) | (3 – 9) |
| Duration of Enrollment, months (mean/median) | 16.5/15.6 | 13.4/13.4 |
| Range | (6.1 – 34.1) | (5.7 – 23.7) |
| Abnormal Vision Prior to Study Entry, n (%) | 5 (50) | 13 (29) |
| Vision Loss Events During Studya | – | |
| Time to Event, months (mean/median) | 6.3/4.7 | |
| Range | (3.0 – 14.1) | – |
| Visual acuity, n (%) | 7 (39) | – |
| Visual field, n (%) | 8 (44) | – |
| Both visual acuity/field, n (%) | 3 (17) | – |
18 events of vision loss experienced during the study.
NF1 = Neurofibromatosis type 1.
New onset vision loss during the study was manifested as either VA loss only (39%), VF loss only (44%) or both VA and VF loss (17%). Circumpapillary RNFL thickness at the first study visit was generally lower in the new vision loss group as compared to those eyes with stable vision (Table 2), but only reached statistical significance in the temporal quadrant (P < 0.05) and global average (P < 0.05). Those patients with abnormal vision experienced their vision loss, on average, 26 months (range, 4 – 48 months) prior to study entry. Given the known differences between SD-OCT devices, the analysis was repeated on subjects imaged with the same device. Subjects who experienced vision loss imaged with the hand-held SD-OCT demonstrated a thinner RNFL at the first study visit than those without vision loss in the superior and temporal quadrants (P < 0.05, and P < 0.01, respectively). There was no difference in RNFL thickness at the first study visit between groups in those imaged with the table-top SD-OCT (Spectralis).
Table 2.
Circumpapillary Retinal Nerve Fiber Layer Thickness Measures at Study Entry and Completion in Children with Optic Pathway Gliomas Experiencing New Onset Vision Loss or Stable Vision.
| New Vision Loss (N = 10) |
Stable Vision (N = 45) |
|||
|---|---|---|---|---|
| First visit | Last visit | First visit | Last visit | |
| Global | 84.7 ± 21.0a | 66.5 ± 18.3b | 104.7 ± 30.5 | 103.3 ± 29.5 |
| Superior | 109.7 ± 34.0 | 86.1 ± 31.7b | 131.3 ± 35.5 | 131.7 ± 34.3 |
| Nasal | 65.4 ± 19.9 | 52.4 ± 18.8b | 81.4 ± 27.6 | 78.9 ± 26.5 |
| Inferior | 115.4 ± 25.4 | 89.1 ± 25.5b | 127.4 ± 37.6 | 125.1 ± 36.6 |
| Temporal | 49.4 ± 19.0a | 38.5 ± 16.9b | 76.6 ± 30.1 | 74.8 ± 28.8 |
P < 0.05, between groups
P < 0.01, between groups
The circumpapillary RNFL thickness at the last study visit was significantly lower in the new vision loss group (P < 0.01 for all locations, except for inferior P < 0.05). This finding was confirmed using either SD-OCT device. Since some eyes had reduced circumpapillary RNFL thickness at study entry, the percent change in circumpapillary RNFL from study entry was calculated and those who experienced vision loss demonstrated approximately 20% decline whereas those eyes with stable vision did not demonstrate much change (Table 3).
Table 3.
Percent Change in Circumpapillary Retinal Nerve Fiber Layer Thickness From Study Entry Until Study Completion in Children with Optic Pathway Gliomas Experiencing New Onset Vision Loss or Stable Vision.
| New Vision Loss (N = 10) |
Stable Vision (N = 45) |
|
|---|---|---|
| Global | −21±9 | −1±3 |
| Superior | −21±11 | 0±4 |
| Nasal | −19±14 | −1±6 |
| Inferior | −23±12 | −1±4 |
| Temporal | −20±19 | −1±6 |
Using a threshold of ≥10% decline in circumpapillary RNFL, the number of study visits exceeding this threshold across anatomic location was highest for those eyes experiencing new vision loss (Table 4). Eyes with stable vision during the study did demonstrate visits with a ≥10% decline in circumpapillary RNFL, although most were isolated and occurred in patients with and without abnormal vision prior to study entry. To determine if the decline in quadrant or global average circumpapillary RNFL was consistent, the number of subjects experiencing ≥10% decline on two consecutive visits was also calculated (Table 4). In the vision loss group, the two consecutive visits with circumpapillary RNFL decline had to occur at the time of vision loss. Three patients not experiencing vision loss had 2 or more consecutive visits with circumpapillary RNFL decline in the superior quadrant. One of these patients had an unusually high baseline measure, therefore all subsequent measures were ≥10% lower, but none of visits after the baseline visit varied by more than 4%. Another patient with stable vision demonstrated ≥10% decline in the superior quadrant at two consecutive visits, although visits following this decline returned to normal in the setting of clinical stability, suggesting unidentified segmentation or acquisition factors resulted in increased variability. A third eye with stable vision that demonstrated a decline in superior circumpapillary RNFL experienced VF loss in the contralateral eye from a chiasmal optic pathway glioma, suggesting the decline was indeed real but not enough to manifest as vision loss.
Table 4.
Number of Study Visits Demonstrating a ≥ 10 % Decline in Circumpapillary Retinal Nerve Fiber Layer Thickness in Children with Optic Pathway Gliomas Experiencing New Onset Vision Loss or Stable Vision.
| New Vision Loss (N = 67) |
Stable vision (N =183) |
|||
|---|---|---|---|---|
| Number of Visits with ≥ 10% Declinea | 2 Consecutive Visits ≥ 10% Declinea,b,c | Number of Visits with ≥ 10% Declinea | 2 Consecutive Visits ≥ 10% Declinea,b | |
| Global | 35 (42) | 6 (60) | 1 (1) | 0 (0) |
| Superior | 33 (49) | 6 (60) | 8 (4) | 3 (7) |
| Nasal | 36 (54) | 6 (60) | 20 (11) | 1 (2) |
| Inferior | 30 (45) | 5 (50) | 5 (3) | 0 (0) |
| Temporal | 26 (39) | 4 (40) | 14 (8) | 2 (4) |
Number (percent)
Per patient
Occuring at Time of Vision Loss
The diagnostic ability to detect vision loss was calculated across anatomic sector (Table 5). Global average and the inferior quadrant demonstrated the best positive and negative predictive values. When two or more quadrants demonstrated a ≥10% decline in circumpapillary RNFL on two consecutive visits, the diagnostic ability was excellent (Table 5, bottom panel).
Table 5.
Diagnostic Ability of Detecting Vision Loss Using a Threshold of ≥ 10 % Decline in Circumpapillary Retinal Nerve Fiber Layer Thickness on Two Consecutive Visits in Children with Optic Pathway Gliomas.
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
|---|---|---|---|---|
| Global | 60% | 100% | 100% | 92% |
| Superior | 60% | 93% | 66% | 91% |
| Nasal | 60% | 98% | 86% | 92% |
| Inferior | 50% | 100% | 100% | 90% |
| Temporal | 40% | 96% | 67% | 88% |
| ≥ 2 Quadrants with ≥ 10% Decline on 2 Consecutive Visits | 70% | 100% | 100% | 94% |
The Kaplan-Meier analysis (Figure 1) revealed an average time until vision loss was 6.3 months with the last event occurring 14.1 months after study enrollment. The magnitude of circumpapillary RNFL decline in eyes experiencing vision loss varied by anatomic sector (Figure 2). Although not analyzed, an additional 8 events of vision loss occurred after the first event of vision loss (filled symbols, Figure 2). One patient eye with an isolated optic nerve glioma demonstrated an increase in circumpapillary RNFL thickness at the time of vision loss in all sectors except the temporal. This increase in circumpapillary RNFL thickness was sustained for approximately 6 months until it declined below baseline measures.
Figure 1.
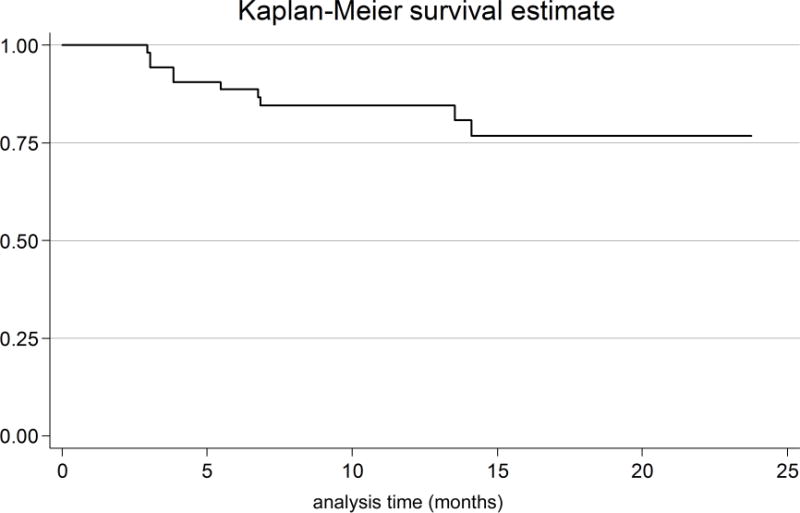
Kaplan-Meier survival curve of the time from study entry until the first event of vision loss in children with optic pathway gliomas. Ten of 55 patient eyes experienced a new event of vision loss. The average time until the first event of vision loss occurred at 6.3 months (range 3 – 14.1 months) after study entry.
Figure 2.
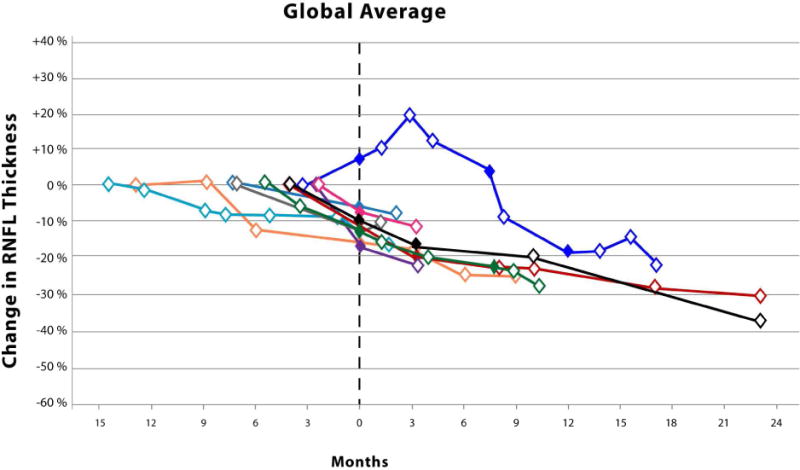
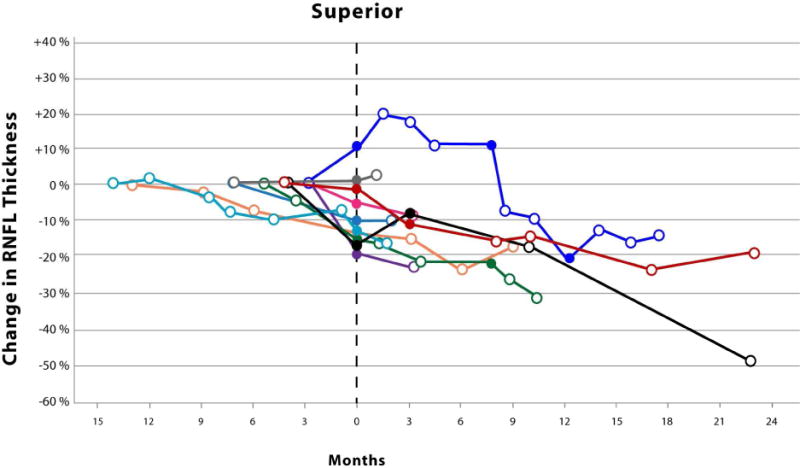
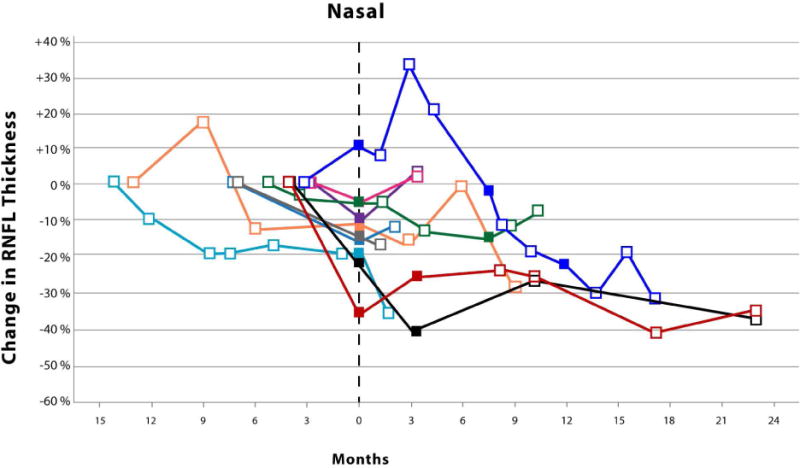
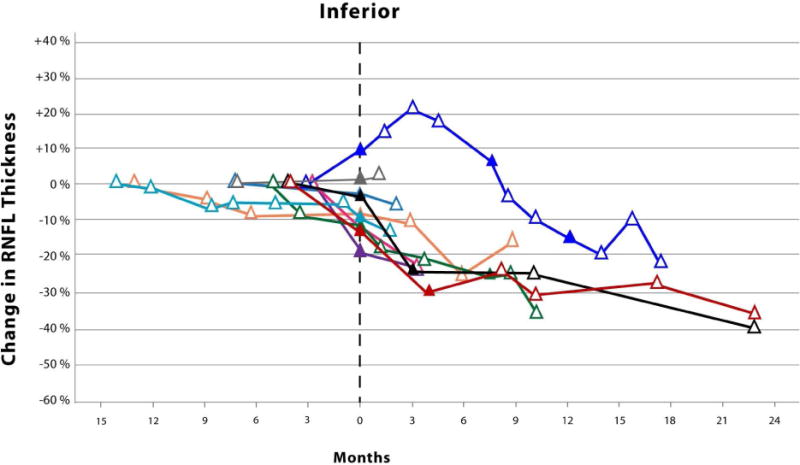
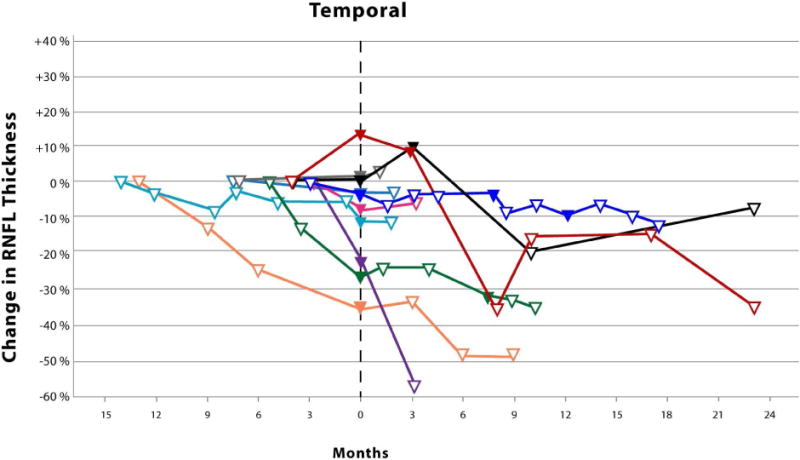
Scatter plots with connecting lines representing the percent change in circumpapillary retinal nerve fiber layer thickness (y-axis), as measured by spectral domain optical coherence tomography, over time (x-axis) in children experiencing vision loss from their optic pathway gliomas. Each colored symbol represents a study visit for a unique study eye. Events of vision loss are noted by a filled symbol. The first event of vision loss experienced during the study is standardized to month zero. Open symbols to the left of month zero represent the first study visit and any subsequent visits when the patient demonstrated stable vision. Symbols to the right of month zero represent subsequent study visits with (closed symbol) or without (open symbol) events of vision loss. Each scatter plot represents the percent change in average (Top) and quadrant specific circumpapillary retinal nerve fiber layer thickness (Middle and Bottom).
Unadjusted analysis using a Cox proportional hazards model demonstrated that percent change in all anatomic sectors reached statistical significance (P < 0.01 for all comparisons). The adjusted analysis considering all anatomic sectors together did not reach significance for any predictor (P > 0.05 for all comparisons). Since the change in global average circumpapillary RNFL was the most robust predictor of an event of vision loss, other clinical contributory variables were examined including gender, race, abnormal vision at study entry, tumor location on magnetic resonance imaging, history of treatment with chemotherapy and SD-OCT device, none of which reached significance in unadjusted and adjusted analysis (P > 0.05, all comparisons).
Discussion
By acquiring longitudinal measures of circumpapillary RNFL thickness in a large cohort of patients with optic pathway gliomas, our study demonstrates that a declining circumpapillary RNFL thickness in one or more quadrants or global average is highly predictive of vision loss. Equally important, the absence of a decline in circumpapillary RNFL thickness is highly predictive of stable vision which could be useful to clinicians when determining if a child needs to initiate or defer treatment of the optic pathway glioma—especially in the setting of new or worsening magnetic resonance imaging findings.
Based on our previously published studies examining intra- and intervisit reproducibility of SD-OCT in children with optic pathway gliomas, we designated a ≥10% decline in circumpapillary RNFL as the threshold indicative of significant change.29,30 While this threshold produced excellent positive and negative predictive values, a number of factors likely contributed to the modest sensitivities for detecting vision loss (Table 5). The sensitivity of the global circumpapillary RNFL only reached 60%, but three of the four patients not meeting threshold had an 8–9% decline. More importantly, these same three patients demonstrated a ≥10% decline of circumpapillary RNFL in either the superior or inferior quadrant during their event of vision loss, reinforcing that some patients may only experience a detectable circumpapillary RNFL decline in a specific quadrant. Determining which quadrant will be most vulnerable to damage from these tumors is challenging as most have engulfed the optic pathways rather than extrinsically compressing one specific region.20 Another likely factor influencing the magnitude of circumpapillary RNFL change required to experience vision loss is the patient’s pre-symptomatic circumpapillary RNFL thickness. Similar to the “tipping point” described in glaucoma,14 a greater amount of circumpapillary RNFL decline may need to occur in patients with normal circumpapillary RNFL thickness whereas those with already reduced circumpapillary RNFL thickness may require less of a decline to experience vision loss. To account for the variability in baseline circumpapillary RNFL thickness, we used a percent change rather than absolute change as our threshold. Furthermore, a percent change in circumpapillary RNFL is intuitive and a much more practical calculation during in a busy clinic, as compared to analyzing slope values from a regression model.
Surprisingly, one patient with a sporadic glioma isolated to the optic nerve demonstrated increases in circumpapillary RNFL thickness up to 15–20% in multiple quadrants at the onset of vision loss that was sustained over many study visits (Figure 2). No disc edema was visualized on ophthalmoscopy in this patient who had a previous history of vision loss and optic nerve pallor. The loss of vision prompted the initiation of chemotherapy with vinblastine. The increased circumpapillary RNFL thickness eventually declined as he experienced additional events of vision loss. None of the patients experiencing vision loss during the study demonstrated similar increases of circumpapillary RNFL thickness prior to or after losing vision. However, a second patient with a sporadic glioma isolated to the optic nerve also demonstrated similar increases in circumpapillary RNFL thickness across multiple quadrants, but they did not experience vision loss nor was optic disc edema visualized. This patient was being with treated with bevacizumab due to a decline in vision prior to study enrollment. Twenty-nine percent of the eyes in the stable vision cohort had isolated optic nerve gliomas, 85% of which were due to Neurofibromatosis type 1. Increased circumpapillary RNFL thickness during episodes of vision loss may be specific to patients with sporadic gliomas isolated to the optic nerve, although it is conceivable that this could also occur in children with Neurofibromatosis type 1. It is plausible that axonal stasis produced the relatively small increase in circumpapillary RNFL thickness (e.g., less than 20 microns) without appreciable optic disc edema, especially in these subjects with preexisting optic nerve pallor.
Longitudinal studies of circumpapillary RNFL decline have focused primarily on patients with glaucoma. One of the earliest studies demonstrated an approximately 12% decline in average circumpapillary RNFL in patients experiencing vision loss from glaucoma.34 The threshold of a 10% decline used in our study was likely sufficient to detect new vision loss as many patients had pre-existing vision loss and circumpapillary RNFL decline prior to study entry. More recent longitudinal studies using SD-OCT report a relatively modest rate of circumpapillary RNFL decline (i.e., 2 microns per year) in glaucoma patients experiencing vision loss compared to patients with stable vision.15,35 Comparison of rates and magnitude of circumpapillary RNFL decline between patients with glaucoma and optic pathway gliomas is problematic since most glaucoma studies use new VF loss as an indicator of disease progression while patients in our study experienced VA and or VF loss. Furthermore, the mechanism of axonal loss in glaucoma is different from optic pathway gliomas, thus it is reasonable to believe that the rate of circumpapillary RNFL decline would also be different.15,35
An important factor in establishing the structure function correlation is determining how closely the decline in circumpapillary RNFL thickness and vision loss occur in time. Patients with optic pathway gliomas typically undergo magnetic resonance imaging and visual exams every 3 to 6 months depending on their clinical and imaging features, so some of our patients had more frequent study visits than others. For example, one patient had their baseline visit 6 months prior to their event of vision loss, thereby making it difficult to determine how closely the decline in circumpapillary RNFL and vision loss are coupled. In order to better determine the timing between circumpapillary RNFL decline and vision loss, future studies would need to require identical exam frequencies for all study patients. Furthermore, the variability and accuracy of visual acuity testing, especially Teller grating acuity, in young children may also complicate our understanding of the structure function relationship. Based on our results and clinical experience, SD-OCT imaging and vision examinations might be feasible every 2 months, especially for patients with additional features concerning for progression of disease.
One limitation of our study, as mentioned above, was the variable frequency of study visits. Patient study visits occurred during their clinical visit, therefore the frequency can vary depending on clinical recommendations from other providers, patient compliance and patient willingness to participate in a research study without any direct clinical benefit. Another limitation is the isolated visits demonstrating a decline in circumpapillary RNFL thickness in the stable vision group. It is likely that some subjects had greater measurement variability due to a relatively thin circumpapillary RNFL. Hopefully improvements in image acquisition and segmentation algorithms could decrease measurement variability. It is conceivable that some patients were experiencing subtle amounts of vision loss and or circumpapillary RNFL decline prior to study entry, thereby lowering their baseline measures. Only 1 patient did not have an examination by the study team prior to study enrollment. At enrollment, this patient had a comprehensive examination that was completely normal which included reliable automated perimetry. The potential variability of vision testing results in children, along with the lower baseline circumpapillary RNFL thickness at study entry in the new onset vision loss group, raises the question whether this variability influenced the study results. Fortunately, the new onset vision loss group averaged two study visits prior to their first event of vision loss, thus decreasing the likelihood that testing variability at baseline had a significant influence on the results. Lastly, the relative small number of subject eyes experiencing vision loss limits the statistical power to determine the influence of other variables on our study results.
The primary reason to treat optic pathway gliomas with chemotherapy or biologic agents is to preserve vision, as these tumors have excellent survival rates and few other major disease related morbidities.20 Despite the poor relationship between change in tumor size and vision outcomes,21,36,37 therapeutic clinical trials continue to use radiographic changes (e.g., increased tumor size or contrast enhancement on magnetic resonance imaging) as the primary outcome measure instead of a more clinically relevant metrics such as VA or VF.23,24,38 The omission of VA or VF as primary outcome measure in pediatric optic pathway gliomas clinical trials is likely due a number of factors. Unlike adult studies where all subjects can complete the same well established VA task (i.e., ETDRS charts), pediatric optic pathway gliomas clinical trials enroll children of all ages, thereby eliminating the opportunity to use the same developmentally appropriate test across the entire study population. Furthermore, the ability to cooperate and reliably complete age appropriate VA testing is variable in young children with optic pathway gliomas.25 The results of our current study suggests that percent change in circumpapillary RNFL thickness could be a valuable outcome measure in a clinical trial since it provides a clinically relevant and reliable quantitative outcome that is not influenced by effort or cooperation, and is very amenable to longitudinal statistical analysis. Furthermore, using circumpapillary RNFL measures as a surrogate marker for vision would be most helpful when young children are unable to cooperate or provide quantitative VA or VF testing. Since a large portion of optic pathway gliomas secondary to Neurofibromatosis type 1 do not need treatment, a stable circumpapillary RNFL could provide further reassurance that the tumor is not damaging the visual pathway and that treatment is not necessary. Despite the promising results from our current and past OCT studies, we do not recommend making treatment decisions based solely on OCT results.26,39
In conclusion, longitudinal measures in circumpapillary RNFL thickness demonstrating a decline of ≥10% have excellent positive and negative predictive value for vision loss. SD-OCT measures of circumpapillary RNFL thickness could be helpful in the management of children with optic pathway gliomas, especially in children unable to cooperate with VA or VF testing.
Acknowledgments
Funding/Support: This article was supported by grants from the National Eye Institute/National Institutes of Health, Bethesda, Maryland (K23-EY022673, R.A.A.; R01-EY013178, H.I., J.S.S.; and P30-008098, H.I., J.S.S.), the National Center for Advancing Translational Sciences/National Institutes of Health, Bethesda, Maryland (UL1TR000075, A.C.), Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health, Bethesda, Maryland (P30 HD040677, A.C.), The Gilbert Family Neurofibromatosis Institute, Washington, DC (R.A.A., R.J.P.), The Eye and Ear Foundation of Pittsburgh, Pittsburgh, Pennsylvania (H.I., J.S.S.), and an unrestricted grant from Research to Prevent Blindness, New York, New York (H.I., J.S.S.).
Other Acknowledgements: The authors would like to thank Graham Quinn, MD, MSCE (Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania) for assistance with developing pediatric visual acuity/visual field protocols, study design and review of the manuscript.
Biography

Dr. Robert A. Avery completed his Neuro-ophthalmology fellowship at the Children’s Hospital of Philadelphia/University of Pennsylvania and a Master’s degree in Clinical Epidemiology at the University of Pennsylvania. Dr. Avery is an assistant professor of Neurology, Ophthalmology and Pediatrics at Children’s National Medical Center in Washington DC where he has a dedicated pediatric neuro-ophthalmology practice and clinical research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Schuman receives royalties for intellectual property licensed by Massachusetts Institute of Technology and Massachusetts Eye and Ear Infirmary to Carl Zeiss Meditec (Dublin, California). The remaining authors have no financial disclosures or potential conflicts of interest.
References
- 1.Kotowski J, Wollstein G, Ishikawa H, Schuman JS. Imaging of the optic nerve and retinal nerve fiber layer: An essential part of glaucoma diagnosis and monitoring. Surv Ophthalmol. 2014;59(4):458–467. doi: 10.1016/j.survophthal.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh EA, Marrie RA, Reginald YA, et al. Functional-structural correlations in the afferent visual pathway in pediatric demyelination. Neurology. 2014;83(23):2147–2152. doi: 10.1212/WNL.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghasia FF, El-Dairi M, Freedman SF, Rajani A, Asrani S. Reproducibility of spectral-domain optical coherence tomography measurements in adult and pediatric glaucoma. J Glaucoma. 2015;24(1):55–63. doi: 10.1097/IJG.0b013e31829521db. [DOI] [PubMed] [Google Scholar]
- 4.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49(5):1879–1885. doi: 10.1167/iovs.07-1127. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal D, Tan O, Huang D, Sadun AA. Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(8):4539–4545. doi: 10.1167/iovs.11-9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112(1):120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Barboni P, Savini G, Cascavilla ML, et al. Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am J Ophthalmol. 2014;158(3):628–636. doi: 10.1016/j.ajo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Waldman AT, Hiremath G, Avery RA, et al. Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Multiple Scler Relat Disord. 2013;3(3):326–334. doi: 10.1016/j.msard.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyer LA, Galetta K, Wilson J, et al. Analysis of the visual system in Friedreich ataxia. J Neurol. 2013;260(9):2362–2369. doi: 10.1007/s00415-013-6978-z. [DOI] [PubMed] [Google Scholar]
- 10.Galetta KM, Calabresi PA, Frohman EM, Balcer LJ. Optical coherence tomography (OCT): imaging the visual pathway as a model for neurodegeneration. Neurotherapeutics. 2011;8(1):117–132. doi: 10.1007/s13311-010-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinto LM, Costa EF, Melo LA, Jr, et al. Structure-Function Correlations in Glaucoma Using Matrix and Standard Automated Perimetry Versus Time-Domain and Spectral-Domain OCT Devices. Invest Ophthalmol Vis Sci. 2014;55(5):3074–3080. doi: 10.1167/iovs.13-13664. [DOI] [PubMed] [Google Scholar]
- 12.Mwanza JC, Durbin MK, Budenz DL, et al. Glaucoma diagnostic accuracy of ganglion cell-inner plexiform layer thickness: comparison with nerve fiber layer and optic nerve head. Ophthalmology. 2012;119(6):1151–1158. doi: 10.1016/j.ophtha.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros FA, Zangwill LM, Bowd C, Mansouri K, Weinreb RN. The Structure and Function Relationship in Glaucoma: Implications for Detection of Progression and Measurement of Rates of Change. Invest Ophthalmol Vis Sci. 2012;53(11):6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollstein G, Kagemann L, Bilonick RA, et al. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br J Ophthalmol. 2012;96(1):47–52. doi: 10.1136/bjo.2010.196907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miki A, Medeiros FA, Weinreb RN, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014;121(7):1350–1358. doi: 10.1016/j.ophtha.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wessel JM, Horn FK, Tornow RP, et al. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(5):3613–3620. doi: 10.1167/iovs.12-9786. [DOI] [PubMed] [Google Scholar]
- 17.Banitt MR, Ventura LM, Feuer WJ, et al. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci. 2013;54(3):2346–2352. doi: 10.1167/iovs.12-11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porciatti V, Ventura LM. Retinal ganglion cell functional plasticity and optic neuropathy. J Neuroophthalmol. 2012;32(4):354–358. doi: 10.1097/WNO.0b013e3182745600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47(9):3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31(3):269–278. doi: 10.1097/WNO.0b013e31822aef82. [DOI] [PubMed] [Google Scholar]
- 21.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55(6):1083–1088. doi: 10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 22.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol. 2012;14(6):790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81(21 Suppl 1):S15–24. doi: 10.1212/01.wnl.0000435745.95155.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110(1):1–7. doi: 10.1007/s11060-012-0944-y. [DOI] [PubMed] [Google Scholar]
- 25.Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54(2):1034–1038. doi: 10.1167/iovs.12-11385. [DOI] [PubMed] [Google Scholar]
- 26.Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthal. 2014;132(3):265–271. doi: 10.1001/jamaophthalmol.2013.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–549. doi: 10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch TM, Gutmann DH. Neurofibromatosis 1. Neurol Clin. 2002;20(3):841–865. doi: 10.1016/s0733-8619(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 29.Rajjoub RD, Trimboli-Heidler C, Packer RJ, Avery RA. Reproducibility of retinal nerve fiber layer thickness measures using eye tracking in children with nonglaucomatous optic neuropathy. Am J Ophthalmol. 2015;159(1):71–77. doi: 10.1016/j.ajo.2014.09.029. e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avery RA, Cnaan A, Schuman JS, et al. Reproducibility of circumpapillary retinal nerve fiber layer measurements using handheld optical coherence tomography in sedated children. Am J Ophthalmol. 2014;158(4):780–787. doi: 10.1016/j.ajo.2014.06.017. e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein DM, Ishikawa H, Hariprasad R, et al. A new quality assessment parameter for optical coherence tomography. Br J Ophthalmol. 2006;90(2):186–190. doi: 10.1136/bjo.2004.059824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46(6):2012–2017. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123(4):464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu G, Weinreb RN, Leung CK. Retinal nerve fiber layer progression in glaucoma: a comparison between retinal nerve fiber layer thickness and retardance. Ophthalmology. 2013;120(12):2493–2500. doi: 10.1016/j.ophtha.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol. 2007;9(4):430–437. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opocher E, Kremer LCM, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: A systematic review. Eur J Cancer. 2006;42(12):1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014;116(2):341–347. doi: 10.1007/s11060-013-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avery RA, Rajjoub RD, Trimboli-Heidler C, Waldman AT. Applications of optical coherence tomography in pediatric clinical neuroscience. Neuropediatrics. 2015;46(2):88–97. doi: 10.1055/s-0035-1549098. [DOI] [PMC free article] [PubMed] [Google Scholar]


