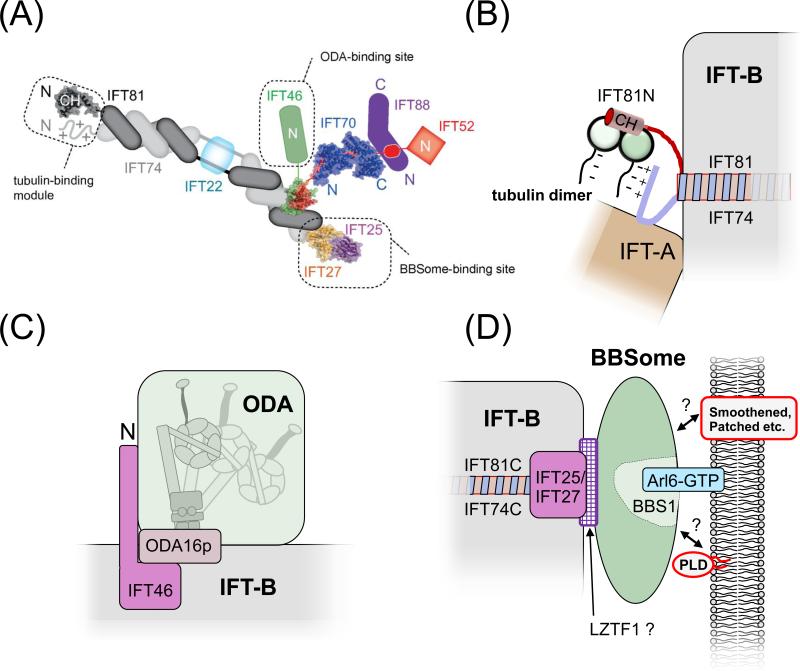
Figure 4. Cargo binding by IFT.
(A) Schematic presentation of the IFT-B core complex. The crystal structures of partial and complete IFT proteins are included where available and the putative cargo binding sites are indicated. Note that the exact positions of IFT27/25 and IFT52/46 on the C-terminal part of IFT74/81 are unknown. Reprinted in modified form from reference [50] with permission of the authors and journal. ©1967 Taschner et al. Journal of Cell Biology. 207:269-282. doi:10.1083/jcb.201408002.
(B) Tubulin binding site. The N-terminal domain of IFT81 forms a CH domain, which binds tubulin dimers. This interaction is stabilized by the N-terminal domain of IFT74, which is positively charged and might interact with the glutamate-rich C-terminal domain of β-tubulin [53].
(C) ODA binding site. Cells expressing an N-terminally truncated IFT46 fail to assemble ODAs onto the axoneme. Mutants in ODA16p have a similar phenotype and ODA16p interacts with IFT46 suggesting that ODA16p stabilizes the interaction of IFT46 and ODAs allowing them to travel on IFT trains [87, 88].
(D) BBSome binding site. Mutant analysis revealed that IFT25/27 are required for the export of BBSomes from cilia suggesting that these IFT proteins form a BBSome binding site. LZTF1 accumulates in Ift27−/− cilia but not those of Bbs−/− mutants and might mediate BBSome binding to IFT27/27. BBS3/Arl6 binds to BBS1 and recruits BBSomes to membranes [69, 103]. The BBSome might function as a cargo adapter allowing various transmembrane and membrane-associated proteins to attach to IFT for ciliary import or export [23, 49].