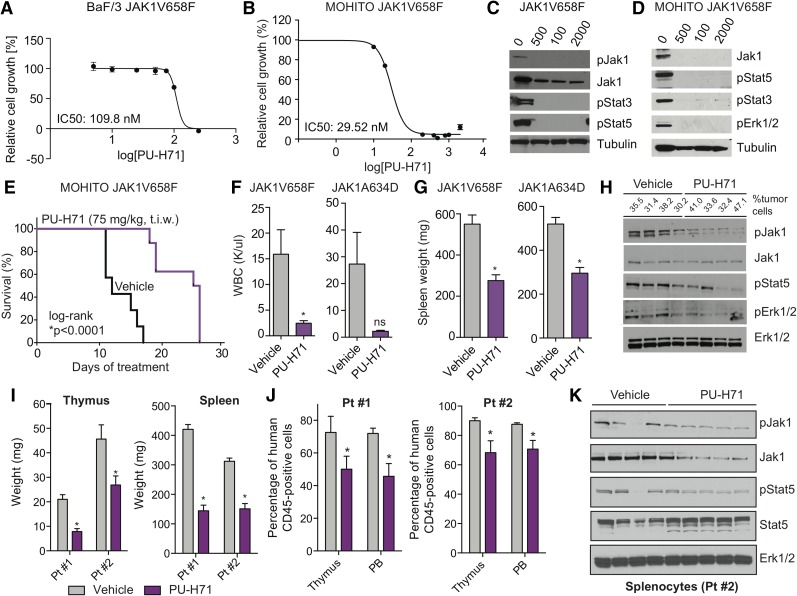
Figure 1.
JAK-mutant ALL cell lines and diseased mice are sensitive to HSP90 inhibition. (A-B) BaF/3 cells (A) and MOHITO cells (B) expressing the ALL-associated JAK1V658F mutation are highly sensitive to growth inhibition by PU-H71. In each case, IC50 values are indicated. Relative cell growth compared with DMSO-treated control is shown. Inhibitor concentrations are plotted in logarithmic scale. (C-D) Western blots reveal a dose-dependent inhibition of JAK1, JAK2, and signaling intermediates in the JAK-STAT pathway after treatment with PU-H71–isogenic BaF/3 (C) and MOHITO (D) cell lines. (E) Cytokine-independent MOHITO cells expressing the activating JAK1V658F mutation were injected (tail vein) into lethally irradiated syngeneic mice. Leukemic mice treated with PU-H71 (75 mg/kg, 3 times per week [t.i.w.], i.p.) survived significantly longer compared with vehicle control mice (vehicle, n = 7; PU-H71, n = 8; *P < .0001, log-rank test). (F-G) Treatment of MOHITO JAK1V658F- and JAK1A634D-transplanted mice treated with PU-H71 significantly reduced WBC (K/μL) (F) and spleen size (G) compared with vehicle-treated controls (*P < .05, Student t test, n = 7 per group). (H) Protein analysis of whole-cell lysates prepared from splenocytes of PU-H71 (single dose, 75 mg/kg, 16 hours prior to tissue harvest) and vehicle-treated JAK1V658F-diseased mice showing that HSP90 inhibition causes degradation of phosphorylated JAK1 in vivo and concomitant inhibition of downstream signaling proteins Stat5 and Erk1/2. Tubulin is shown as loading control. Tumor burden (percentage of GFP-positive cells) in the spleen at time of tissue harvest is shown. (I) Thymus (*P < .05, Student t test) and spleen (*P < .05, Student t test) size was significantly smaller in PU-H71–treated mice compared with vehicle control; n = 7-12 in each group. (J) PU-H71–treated mice had lower numbers of leukemic human CD45-positive cells in the thymus and PB; n = 7-13 in each group. Data from 2 patient-derived xenograft models are shown. (K) Protein analysis of whole-cell lysates from splenocytes of vehicle- and PU-H71–treated mice (Pt #2) showing that HSP90 inhibition causes degradation of total and phosphorylated Jak1 in vivo and inhibition of downstream signaling target Stat5. Erk1/2 is shown as independent loading control. DMSO, dimethylsulfoxide; Erk, extracellular signal-regulated kinase; GFP, green fluorescent protein; IC50, 50% inhibitory concentration; i.p., intraperitoneally; ns, not significant; p, phosphorylated form; PB, peripheral blood; Pt, patient; WBC, white blood cell count.