Abstract
Objectives
The aim of this study was to determine the relationship between the intensity of snoring and severity of sleep apnea using Watch-PAT (peripheral arterial tone) 100.
Methods
A total of 404 patients (338 males and 66 females) who underwent home-based portable sleep study using Watch-PAT 100 for obstructive sleep apnea (OSA) from January 2009 through December 2011 were included in this study. Subjects were divided into 4 groups; no OSA (PAT apnea hypopnea index [pAHI]<5/hour), mild OSA (5≤pAHI<15/hour), moderate OSA (15≤pAHI<30/hour), or severe OSA groups (pAHI≥30/hour). Mean snoring intensity and percent sleep time with snoring intensity greater than 40, 50, and 60 dB were measured by Watch-PAT 100. Correlations of these parameters with apnea hypopnea index (AHI), respiratory disturbance index (RDI), and oxygen desaturation index were assessed.
Results
The mean age and body mass index were 46.5±14.8 years and 24.7±3.4 kg/m2, respectively. Mean AHI and RDI were 16.5±15.3/hour and 20.8±14.3/hour, respectively. The mean snoring intensity in the no, mild, moderate, and severe OSA groups was 44.0±2.7, 45.4±6.0, 47.7±5.0, and 50.5±5.6 dB, respectively (P<0.001). There was a positive correlation between snoring intensity and pAHI or PAT RDI (pRDI) (r=0.391 and r=0.385, respectively, both P<0.001). There was also a positive correlation between percent sleep time with the snoring intensity greater than 50 dB and pAHI or pRDI (r=0.423 and r=0.411, respectively, both P<0.001).
Conclusion
This study revealed that the intensity of snoring increased with the severity of sleep apnea, which suggests that the loudness of snoring might be an indicator of the severity of OSA.
Keywords: Snoring, Obstructive Sleep Apnea, Desaturation, Watch-PAT 100
INTRODUCTION
Snoring is the major and most common manifestation of obstructive sleep apnea (OSA) [1,2] and patients usually visit the outpatient clinic due to snoring rather than OSA. Snoring is caused by narrowing or obstruction of the upper airway during sleep.
Recently, a large population-based study showed that snoring intensity was correlated with the severity of OSA using polysomnography [3]. However, there have been relatively few studies on the correlation of snoring intensity with OSA severity. Furthermore, the correlation has never been studied using portable monitoring devices, such as Watch-PAT (peripheral arterial tone) 100 (Itamar, Caesarea, Israel). The Watch-PAT 100 is an ambulatory 4-channel portable monitoring device that is currently approved by the Centers for Medicare and Medicaid Services for the diagnosis of OSA and its usage is increasing these days. The WatchPAT can detect snoring intensity and OSA severity parameters, such as apnea hypopnea index (AHI), respiratory disturbance index (RDI), and oxygen desaturation index (ODI) using PAT signals [4,5,6]. The WatchPAT has several advantages compared with PSG as it is cheaper, can be easily performed, and can be performed unattended during data acquisition after it was approved by the U.S. Food and Drug Administration in 2001. The data can also be interpreted automatically so that the interpersonal interpretation difference does not exist. Due to the above advantages, its use has gradually increased worldwide.
In this study, we tried to evaluate the correlation between snoring parameters including snoring intensity and respiratory parameters using a home-based portable monitoring device, Watch-PAT 100.
MATERIALS AND METHODS
Subjects
From January 2009 through December 2011, 404 subjects (338 males) who visited the Sleep Center at Seoul National University Bundang Hospital were retrospectively included. All the subjects underwent a home-based portable sleep study using Watch-PAT 100. Patients with the following criteria could not undergo Watch-PAT 100 and were excluded from this study. The exclusion criteria for this study were as follows: (1) history of peripheral vasculopathy or neuropathy, and/or autonomic nervous system dysfunction, cardiac problems (nonsinus cardiac rhythm, permanent pacemaker), severe lung disease, severe diabetes, bilateral sympathectomy, and use of alpha-adrenergic receptor-blocking agents; (2) severe finger deformity that could affect adequate application of the PAT probe. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Watch-PAT 100
Watch-PAT 100 is a portable monitoring device without specific respiratory channels, and it was approved by the Centers for Medicare and Medicaid Services for the diagnosis of OSA. The principle of Watch-PAT 100 is as follows: Four channels are used to document the following parameters-PAT signal (PAT probe), heart rate (PAT signal), oxyhemoglobin saturation (pulse oximetry), and wrist activity (actigraphy). The mechanisms of Watch-PAT 100 for the detection of respiratory events are as follows [5]: (1) the termination of respiratory disturbances leads to the surge of sympathetic activity that influences digital arterial vasoconstriction; (2) vasoconstriction of the digital vascular bed mediated by alpha-receptors results in attenuation of PAT signal; and (3) PAT signal attenuation, increased heart rate, and oxygen desaturation are analyzed by the automatic computerized algorithm of the Watch-PAT 100 system. The frequency of respiratory events per hour of actigraphy-determined sleep was then calculated.
Intensity of snoring
The snoring intensity was measured by a microphone connected to the Watch-PAT 100 device, and the device calculated and showed the mean intensity of snoring and the percentages of sleep time out of total sleep time during which snoring was louder than 40, 50, or 60 dB. The mean loudness of snoring and the percentage of sleep time were used for analyses.
Severity of OSA
The severity of OSA was determined by the PAT AHI (pAHI), which was calculated by the Watch-PAT 100 software, and subjects were classified into 4 groups according to the pAHI; no OSA (pAHI<5/hour), mild OSA (5≤pAHI<15/hour), moderate OSA (15≤pAHI<30/hour), or severe OSA groups (pAHI≥30/hour).
Statistical analysis
Student t-test was used to analyze the differences among groups and correlation analyses were used to evaluate the correlation between snoring parameters and respiratory parameters. All parametric results were expressed as mean±SD. Statistical significance was assumed at P<0.05 for all parameters.
RESULTS
Demographic characteristics
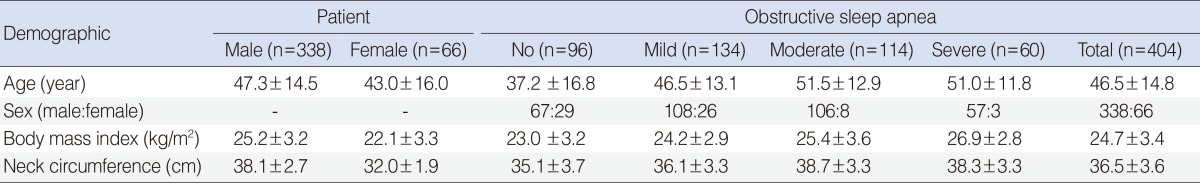
The mean age of all subjects was 46.5±14.8 years (range, 12 to 77 years) and male to female ratio was 338:66, showing male predominance in this series. Males had a higher age, higher body mass index (BMI), and larger neck circumference than females. Age of patients with OSA was higher than that of subjects without OSA (P<0.01), and there was no difference in age according to the severity of OSA. The BMI and neck circumference showed an increasing tendency with increasing severity of OSA (Table 1).
Table 1. Demographics of patients with snoring.
Values are presented as mean±SD.
Polysomnographic data
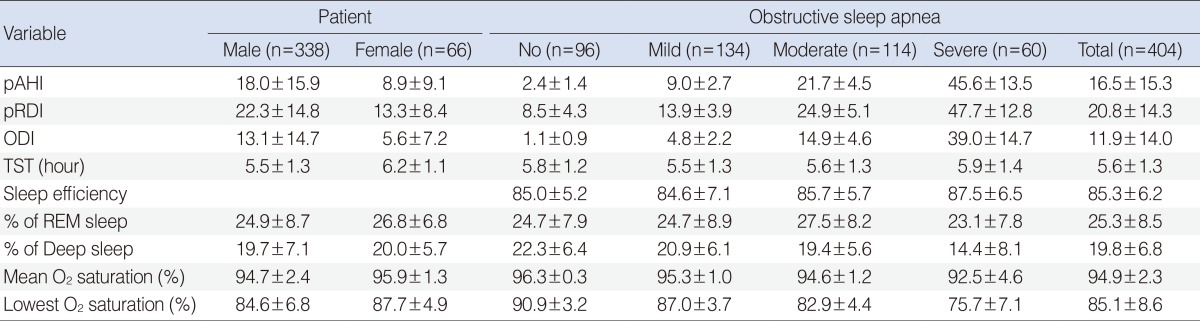
The WatchPAT results are summarized in Table 2. In brief, males had higher pAHI, PAT RDI (pRDI), and ODI, and lower mean and minimal oxygen saturation than females (all, P<0.001). The percentage of REM sleep and deep sleep did not show any difference between males and females (P=0.104 and P=0.721, respectively). According to the severity of OSA, total sleep time and sleep efficiency did not show any difference (P>0.05) and the percentages of deep sleep, mean oxygen saturation, and minimal oxygen saturation were decreased with an increase in OSA severity (all, P<0.001).
Table 2. Polysomnographic findings.
Values are presented as mean±SD.
pAHI, peripheral arterial tone apnea hypopnea index; pRDI, peripheral arterial tone respiratory disturbance index; ODI, oxygen desaturation index; TST, total sleep time, REM, rapid eye movement.
Snoring intensity and severity of OSA
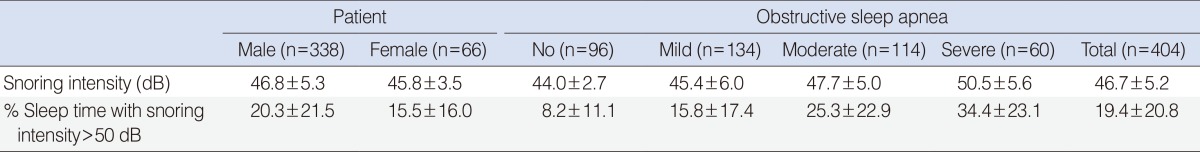
The mean intensity of snoring in no, mild, moderate, and severe OSA groups was 44.0±2.7, 45.4±6.0, 47.7±5.0, and 50.5±5.6 dB, respectively, showing a progressive increase with increasing severity of OSA (P<0.001). The correlation between snoring intensity and percent sleep time with snoring intensity greater than 40, 50, 60, 70, or 80 dB was evaluated, and the percent sleep time with snoring intensity greater than 50 dB was the most correlated with the mean snoring intensity (r=0.884, P<0.001). The percent sleep time with snoring intensity greater than 50 dB also showed a gradual increase with increasing severity of OSA (P<0.001) (Table 3).
Table 3. Snoring intensity and percent sleep time with snoring intensity greater than 50 dB.
Values are presented as mean±SD.
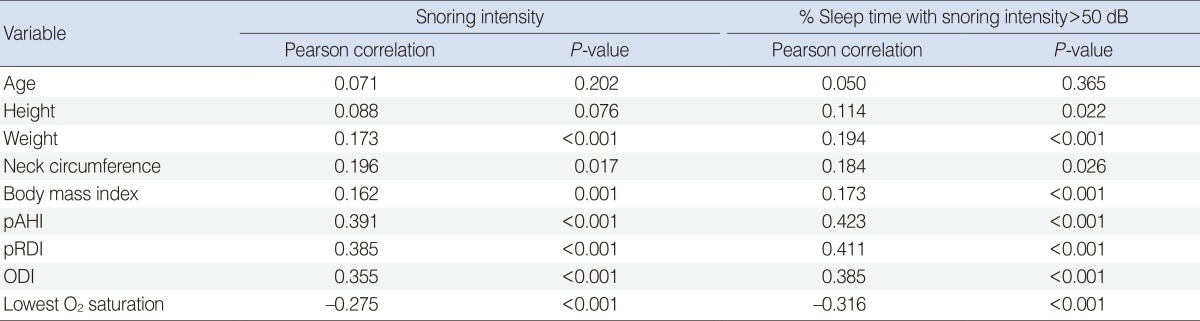
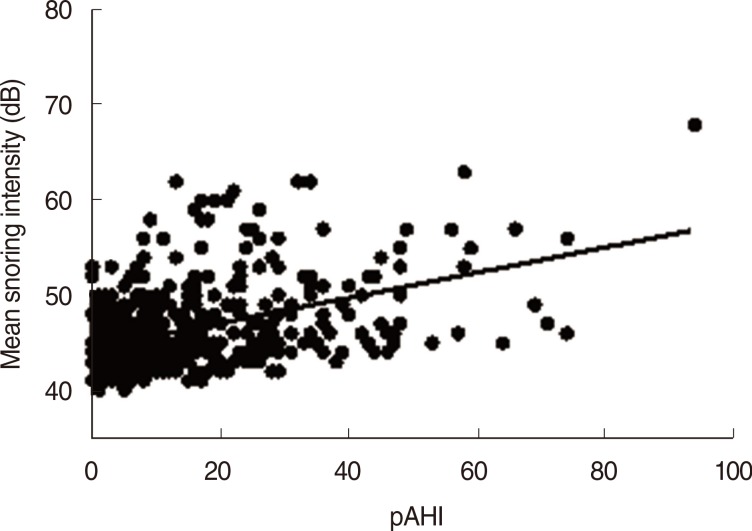
Males tended to have greater snoring intensity and higher percent sleep time with snoring intensity greater than 50 dB than females (P=0.044 and P=0.036, respectively). Further correlation analyses were performed to evaluate the relationship of mean snoring intensity and percent sleep time with snoring intensity greater than 50 dB with multiple parameters, such as age, height, weight, neck circumference, BMI, pRDI, pAHI, ODI, and lowest O2 saturation. All parameters except for age and height showed a significant correlation with the mean snoring intensity and percent sleep time with snoring intensity greater than 50 dB (Table 4). Respiratory parameters such as pAHI, pRDI, and ODI showed more significant correlation coefficients with snoring intensity (r=0.391, r=0.385, and r=0.355, respectively; all, P<0.001) (Fig. 1) and percent sleep time with snoring intensity greater than 50 dB (r=0.423, r=0.411, and r=0.385, respectively; all, P<0.001) than the other parameters such as weight, neck circumference, and BMI (Table 4).
Table 4. Correlation of snoring parameters with respiratory parameters.
pAHI, peripheral arterial tone apnea hypopnea index; pRDI, peripheral arterial tone respiratory disturbance index; ODI, oxygen desaturation index.
Fig. 1. Correlation between peripheral arterial tone apnea hypopnea index (pAHI) and snoring intensity.
DISCUSSION
This study showed that the mean snoring intensity increased with increasing severity of OSA by using the home-based portable sleep monitoring device, Watch-PAT 100. This is the first study using Watch-PAT 100 to demonstrate the correlation between snoring intensity and severity of OSA.
Initially, snoring intensity was generally studied to determine different characteristics of snoring between apneic snorers and nonapneic snorers [7,8]. There have been relatively few studies that show the correlation between snoring intensity and severity of OSA. In 1999, an acoustic study in 1,139 individuals showed that apneic snorers had higher snoring intensity than nonapneic snorers and that noise generated by snoring could disrupt the patient's and bed partner's sleep [9]. Recently, a study showed that the peak intensity of snoring was correlated with AHI in 60 male patients with OSA [10], and a study using a continuous snoring monitoring system suggested that the intensity of snoring sound would allow differentiation of snorers according to OSA severity [11].
Maimon and Hanly [3] were the first to show the correlation between snoring intensity and the severity of OSA using polysomnography in a large population-based study. They showed a positive correlation between AHI and the intensity of snoring (r=0.66, P<0.01), which is consistent with our results. The correlation coefficient was higher than that in our study, which might be attributed to the large sample size and use of level I polysomnography.
The mechanism how the severity of OSA influences the snoring intensity is not well known. Snoring is characterized by high-frequency oscillations of the soft palate, pharyngeal walls, epiglottis, and with the increase in OSA severity, the pressure generated in the airway during apnea might be higher, resulting in higher snoring intensity. In other words, it might be possible that negative pressure generated during apnea might be higher in severe OSA patients such that the snoring intensity might be increased. However, this needs to be clarified with further studies.
Our results provide useful clinical information necessitating a sleep study in patients with heavy snoring, since snoring increases as OSA becomes more severe. Also, this study can be a theoretical basis for a screening device for detection of OSA using snoring intensity, and smartphone application can be an ideal platform for this screening device. Further studies are needed to determine the cutoff value of snoring intensity for predicting OSA. However, since the variability of snoring intensity between subjects was too wide, snoring intensity could not be used as the sole predictor of OSA, and sleep study including Watch-PAT 100 or polysomnography is needed for the final diagnosis.
This study, however, has some limitations. Firstly, this study was performed using a home-based level III portable monitoring device, Watch-PAT 100 instead of gold standard polysomnography. Although this might reduce the significance of our study, the validity of Watch-PAT 100 has already been proven in many studies and our study also provided data consistent with previous studies [5,12,13,14]. Therefore, this could be another study that may prove the validity of Watch-PAT 100 compared to standard polysomnography. Secondly, the number of subjects was relatively small compared with that in a previous study. However, this study included more than 400 subjects, which is one of the largest population-based studies using Watch-PAT 100. Thirdly, we only emphasized the correlation between snoring intensity and severity of OSA, although other variables such as weight, height, neck circumference, and BMI are also related to snoring intensity. Multivariate analysis using all the above variables revealed that respiratory variables including AHI are related to snoring intensity and physical variables such as BMI, height, weight, and neck circumference have lower partial correlation coefficients without statistical significance, showing a strong correlation between severity of OSA and snoring intensity. In spite of all these limitations, this study provided novel information that snoring intensity increases with increasing severity of OSA using a portable device, Watch-PAT 100, and it necessitates the use of sleep studies in patients with heavy snoring.
In conclusion, snoring intensity increased as OSA became more severe in our Watch-PAT 100-based study. Therefore, snoring intensity could be used as one of the indicators to help suspect the diagnosis of sleep apnea.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI15C1524).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM, Guilleminault C, Priest RG, Caulet M. Snoring and breathing pauses during sleep: telephone interview survey of a United Kingdom population sample. BMJ. 1997 Mar;314(7084):860–863. doi: 10.1136/bmj.314.7084.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maimon N, Hanly PJ. Does snoring intensity correlate with the severity of obstructive sleep apnea? J Clin Sleep Med. 2010 Oct;6(5):475–478. [PMC free article] [PubMed] [Google Scholar]
- 4.Pang KP, Gourin CG, Terris DJ. A comparison of polysomnography and the WatchPAT in the diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007 Oct;137(4):665–668. doi: 10.1016/j.otohns.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Choi JH, Kim EJ, Kim YS, Choi J, Kim TH, Kwon SY, et al. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010 Jul;130(7):838–843. doi: 10.3109/00016480903431139. [DOI] [PubMed] [Google Scholar]
- 6.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003 Sep;4(5):435–442. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 7.Pasterkamp H, Schafer J, Wodicka GR. Posture-dependent change of tracheal sounds at standardized flows in patients with obstructive sleep apnea. Chest. 1996 Dec;110(6):1493–1498. doi: 10.1378/chest.110.6.1493. [DOI] [PubMed] [Google Scholar]
- 8.Fiz JA, Abad J, Jane R, Riera M, Mananas MA, Caminal P, et al. Acoustic analysis of snoring sound in patients with simple snoring and obstructive sleep apnoea. Eur Respir J. 1996 Nov;9(11):2365–2370. doi: 10.1183/09031936.96.09112365. [DOI] [PubMed] [Google Scholar]
- 9.Wilson K, Stoohs RA, Mulrooney TF, Johnson LJ, Guilleminault C, Huang Z. The snoring spectrum: acoustic assessment of snoring sound intensity in 1,139 individuals undergoing polysomnography. Chest. 1999 Mar;115(3):762–770. doi: 10.1378/chest.115.3.762. [DOI] [PubMed] [Google Scholar]
- 10.Herzog M, Schieb E, Bremert T, Herzog B, Hosemann W, Kaftan H, et al. Frequency analysis of snoring sounds during simulated and nocturnal snoring. Eur Arch Otorhinolaryngol. 2008 Dec;265(12):1553–1562. doi: 10.1007/s00405-008-0700-2. [DOI] [PubMed] [Google Scholar]
- 11.Fiz JA, Jane R, Sola-Soler J, Abad J, Garcia MA, Morera J. Continuous analysis and monitoring of snores and their relationship to the apnea-hypopnea index. Laryngoscope. 2010 Apr;120(4):854–862. doi: 10.1002/lary.20815. [DOI] [PubMed] [Google Scholar]
- 12.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004 Aug;27(5):923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006 Mar;29(3):367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 14.Onder NS, Akpinar ME, Yigit O, Gor AP. Watch peripheral arterial tonometry in the diagnosis of obstructive sleep apnea: influence of aging. Laryngoscope. 2012 Jun;122(6):1409–1414. doi: 10.1002/lary.23233. [DOI] [PubMed] [Google Scholar]