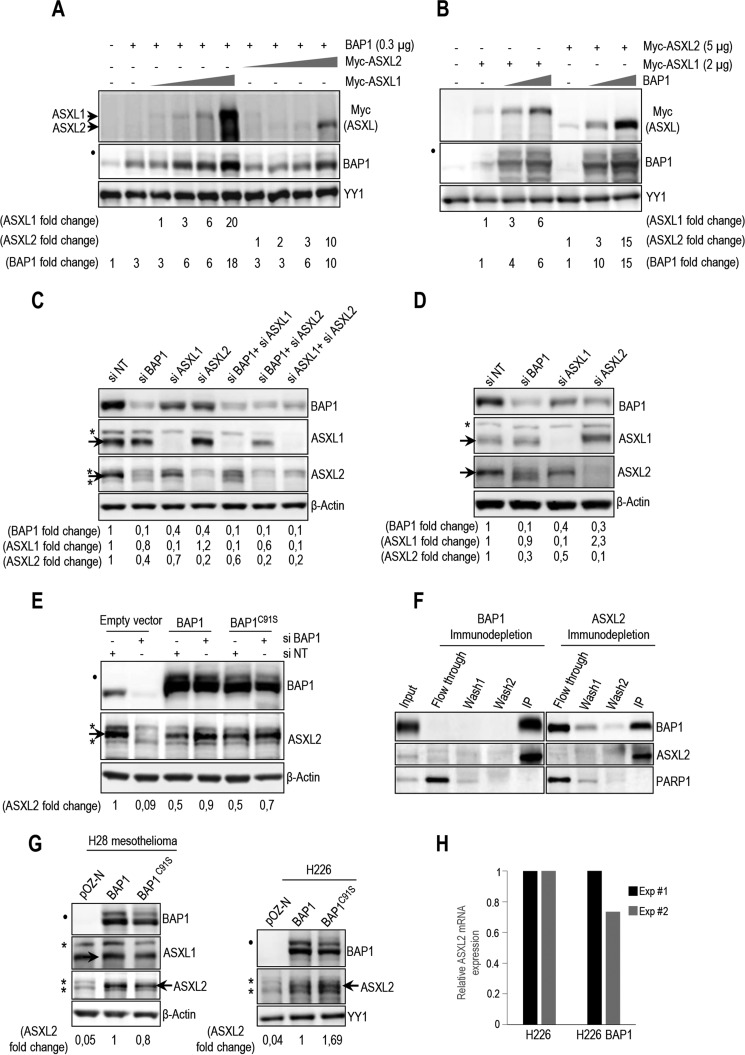
FIGURE 2.
BAP1 and ASXL1/2 are co-regulated, and loss of BAP1 in cancer is concomitant with ASXL2 depletion. A, 293T cells were transfected with BAP1 and increasing amounts of either Myc-ASXL1 (0.5, 1, 2, and 5 μg) or Myc-ASXL2 (0.5, 1, 2, and 5 μg) expression vectors and harvested, 3 days later, for immunoblotting. B, 293T cells were transfected with Myc-ASXL1 or Myc-ASXL2 with increasing amounts of BAP1 (0.3 and 1 μg) vectors and harvested, 3 days later, for immunoblotting. Quantification of band intensity for each protein was conducted relative to the lowest amount of transfected plasmid (A and B). C, protein levels following siRNA depletion of BAP1 and/or ASXL1/2 in U2OS cells. D, protein expression following siRNA depletion of BAP1, ASXL1, and ASXL2 in LF1 human fibroblasts. E, depletion of endogenous BAP1 using siRNA in U2OS cells stably expressing empty vector, siRNA-resistant BAP1 wild type, or siRNA-resistant BAP1 catalytic dead mutant (C91S). Protein levels of BAP1 and ASXL2 were detected by immunoblotting. Quantification of band intensity was conducted relative to the non-target siRNA control (C–E). F, immunodepletion of BAP1 or ASXL2 from HeLa nuclear extracts. The nuclear DNA damage signaling enzyme, PARP1, was used as a control, which mostly remained in the flow-through fraction. G, reconstitution by retroviral infection of H28 mesothelioma and H226 non-small lung carcinoma BAP1-deficient cells with BAP1 or BAP1C91S. Protein levels of BAP1 and mutants were detected by immunoblotting. H, mRNA of ASXL2 in reconstituted H226 cells was quantified by quantitative PCR. The data represent two independent experiments. β-Actin or YY1 are used as protein loading controls. Quantification of band intensity was conducted relative to BAP1-transfected samples (G). The dot and asterisks indicate a monoubiquitinated form of BAP1 (31) and nonspecific bands, respectively (A–E and G).