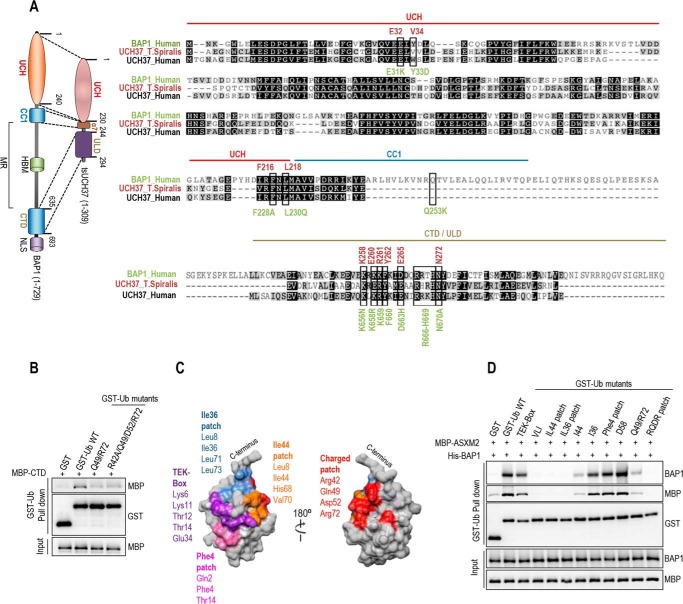
FIGURE 8.
BAP1 CTD is a ubiquitin-interacting domain. A, comparison between BAP1 and UCH37. tsUCH37 of the worm T. spiralis, whose crystal structure was recently reported (38), was aligned with human UCH37 and BAP1. The functionally conserved domains between BAP1 and tsUCH37 are shown in the left panel. The alignment (right panel) shows conserved motifs and residues in the UCH, CC1, and CTD domains. The mutants of BAP1 including the cancer-associated mutants used in Fig. 9 are shown. Note the presence in the CTD of the cancer mutant BAP1R666-H669 with a deletion of the Arg-666 to His-669 amino acids. B, MBP-CTD (3 μg, 40 nm) of BAP1 was subjected to GST-ubiquitin pulldown assay using GST-ubiquitin wild type or its mutant forms (3 μg, 80 nm) (all residues were converted to alanines) and then analyzed by immunoblotting. C, ubiquitin structure showing the various interaction interfaces. D, GST-ubiquitin pulldown assay interaction assays using GST-ubiquitin wild type or its different mutant forms (all residues of each path were converted to alanines) and His-BAP1 with MBP-ASXM2 followed by immunoblotting. The pulldown was done as in Fig. 7F.