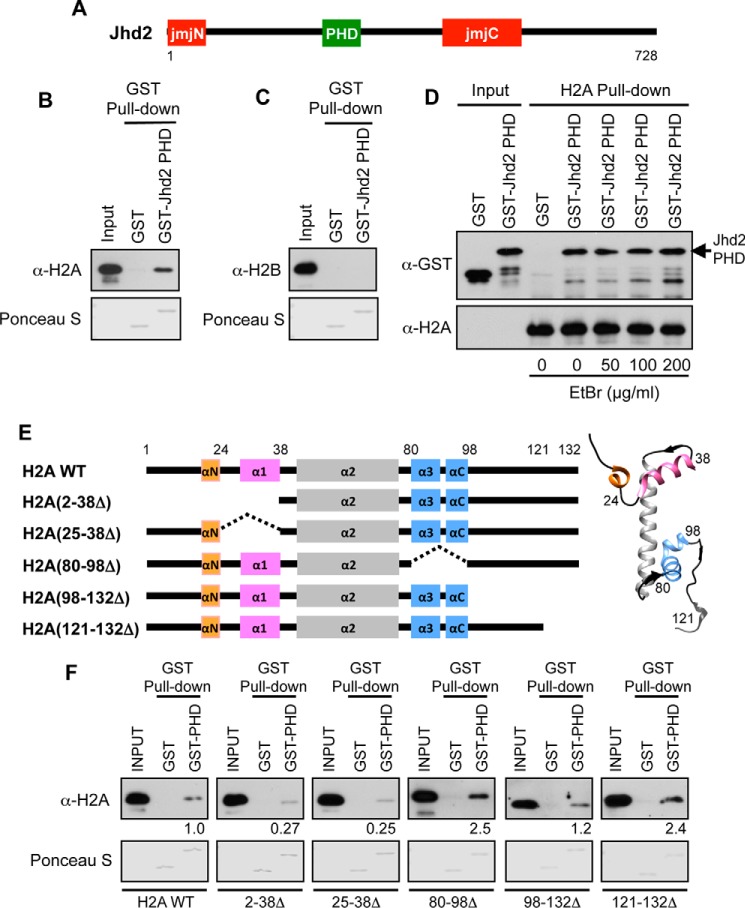
FIGURE 1.
The Jhd2 PHD finger interacts with H2A in vitro. A, schematic representation of Jhd2 with the catalytic JmjN and JmjC domains and the PHD is shown. Numbers denote the first and the last amino acid positions. B and C, recombinant GST alone or GST-tagged Jhd2 PHD finger was incubated with E. coli lysate containing recombinant histones H2A (B) or H2B (C) and subjected to pull-down using glutathione-conjugated beads. After extensive washing, the Jhd2 PHD finger-bound H2A or H2B was eluted in 1× Laemmli sample buffer. E. coli lysates (1%, Input) and eluates (40%) were subjected to Western blotting using α-H2A or α-H2B antibody. The top part of the blot was stained with Ponceau S to show equal loading of GST and GST-tagged PHD finger. D, the α-H2A antibody and protein A-conjugated beads were added to capture the recombinant H2A present in E. coli lysates. After extensive washing, the H2A-bound beads were incubated with the recombinant GST-tagged Jhd2 PHD finger in the absence or in the presence of the indicated concentrations of EtBr. As a negative control, GST alone was incubated with H2A-bound beads. The H2A-bound proteins were eluted from beads using Laemmli sample buffer. Purified GST or GST-Jhd2 PHD finger (1%, Input) and eluates (40%) were subjected to Western blotting using α-GST and α-H2A antibodies. E, schematic representations of histone H2A and its deletion mutants. Helices in H2A (αN, α1, α2, α3, and αC) are also shown. Right, crystal structure of yeast H2A (Protein Data Bank entry 1ID3) with helices colored as in the schematic on the left. F, recombinant GST alone or GST-tagged Jhd2 PHD finger was incubated with the E. coli lysates containing H2A or the deletion mutants, subjected to pull-down using glutathione-conjugated beads, and examined by Western blotting as described for A. Quantitations of the GST-Jhd2 PHD finger interaction with wild-type H2A and with deletion mutants are shown. Blots were quantified by densitometry using ImageJ. The signal for bead-bound histone was normalized to the signal for input control. The signal obtained for a truncated H2A is shown relative to the signal for H2A, which was set as 1.