FIGURE 7.
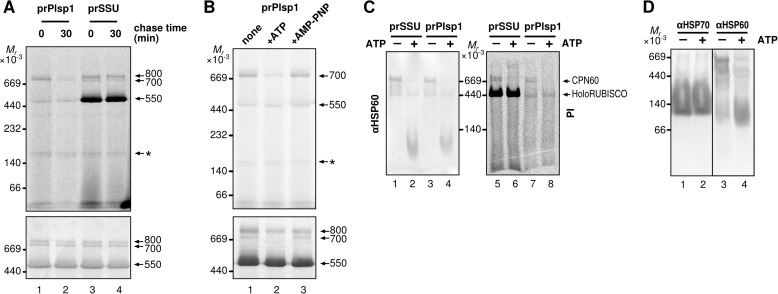
Incorporation of soluble import intermediates into a large oligomeric complex. A, separation of soluble intermediate by BN-PAGE after import-chase. After 10-min import of radiolabeled proteins indicated on top, chloroplasts were treated with thermolysin, reisolated, and subjected to 0- or 30-min chase as described in the legend to Fig. 3D. Chloroplasts were then lysed hypotonically and fractionated by centrifugation. The resultant soluble fraction was separated by BN-PAGE and proteins on the same gel visualized using phosphorimaging (top panel) or by Coomassie Brilliant Blue staining (bottom panel). Three complexes of 800, 700, and 550 kDa are indicated at right with the numbers. An asterisk indicates a nonspecific band. Note that the lane loaded with imported Plsp1 contains a 550-kDa band. This corresponds to the RUBISCO holoenzyme, either as a shadow of the endogenous one, which is abundant, or the one incorporating its large subunit translated from the chloroplast ribosomes with residual radiolabeled amino acids derived from in vitro translation. B, mobility of soluble import intermediate on BN-PAGE after 10-min import followed by various treatments. After 10-min import of radiolabeled prPlsp1, chloroplasts were reisolated, lysed hypotonically, and fractionated by centrifugation. The resultant soluble fraction was further incubated on ice for 10 min with buffer (none), 10 mm ATP or 10 mm AMP-PNP, then separated, and analyzed as described in the legend to A. The 700-kDa complex and the band corresponding to the RUBISCO holoenzyme are indicated at right. C, co-migration of soluble import intermediates with Cpn60 on BN-PAGE. After 10-min import of radiolabeled proteins indicated on top into intact chloroplasts, the soluble fraction was recovered as in A and treated without (−) or with 10 mm ATP (+) for 10 min, separated by BN-PAGE, transferred to a PVDF membrane, and examined by immunoblotting with the antibody against Hsp60 (αHSP60, left panel) or using phosphorimaging (PI, right panel). Cpn60 complex and RUBISCO holoenzyme (HoloRUBISCO) are indicated at right. D, migration of Cpn60 and Hsp70 complexes on BN-PAGE. Soluble chloroplast fractions prepared as in A without import of radiolabeled proteins were incubated without (−) and with (+) 10 mm ATP, separated by SDS-PAGE, transferred to a PVDF membrane, and examined by immunoblotting with antibodies against Hsp70 (αHSP70) or Hsp60 (αHSP60).