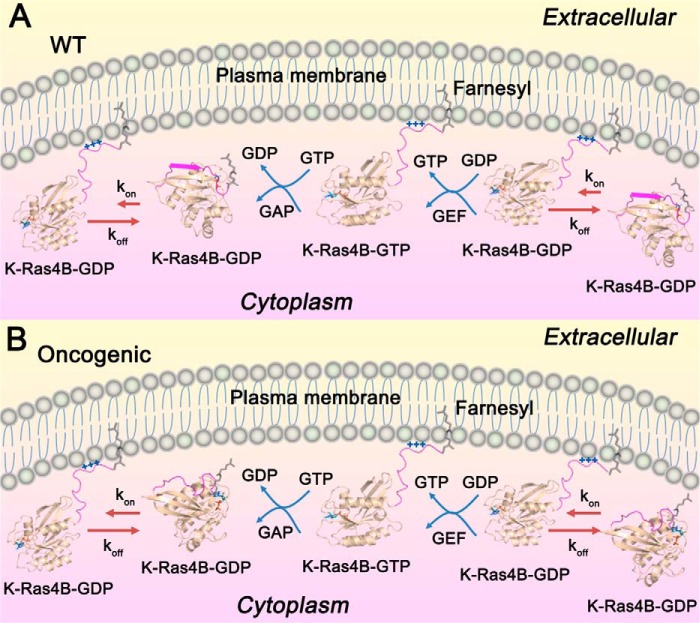
FIGURE 10.
The mechanisms of wild-type and oncogenic K-Ras4B regulation. In resting cells, K-Ras4BWT (A) is predominantly GDP-bound, and the farnesylated HVR of K-Ras4BWT-GDP forms an antiparallel β sheet with the β2 sheet of the catalytic domain. In the cytoplasma pool, K-Ras4BWT-GDP is in a dynamic equilibrium between the released (kon) and unreleased (koff) HVR states (kon ≪ koff), which renders the full-length K-Ras4B-GDPWT primary in the unreleased, autoinhibited form. The released HVR state of K-Ras4BWT-GDP can insert the farnesylated HVR into the PM, where the polybasic stretch of the HVR forms electrostatic interactions with the negatively charged phospholipids in the inner leaflet of PM. At the PM, guanine nucleotide exchanges (GEF) exchange GDP for GTP to produce the active K-Ras4BWT-GTP, which can bind its effectors, e.g. Raf, PI3K, and Ral guanine nucleotide dissociation stimulator (RalGDS), to initiate downstream signaling. GTPase activating proteins (GAP) accelerate GTP→GDP hydrolysis to produce the inactive K-Ras4BWT-GDP, thereby terminating signaling. After disassociation from the PM, K-Ras4BWT-GDP is autoinhibited again by the interactions of its HVR β sheet with the catalytic domain to complete catalytic process. B, In the oncogenic states, the mutations impair the β sheet secondary structure of HVR and weaken the interactions between the HVR and the catalytic domain. This effect shifts the equilibrium to the released HVR state of K-Ras4BMut-GDP and increases the population of the released HVR state of K-Ras4BMut-GDP compared with the wild type. As a result, more population of K-Ras4BMut-GDP can traffic to the PM to recruit its effectors, thereby leading to the increase of downstream signaling associated with oncogenic cell growth.