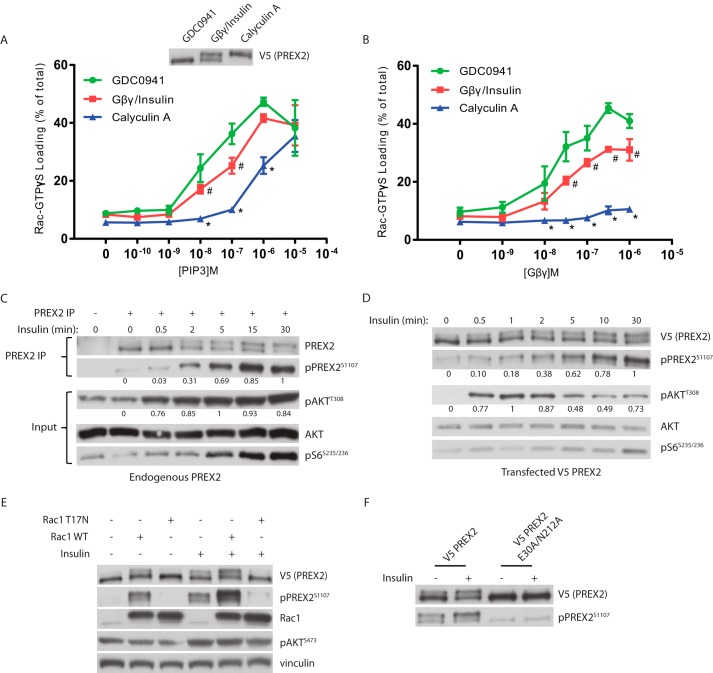
FIGURE 6.
Phosphorylation reduces PREX2 GEF activity and is dependent on Rac1. A and B, V5 PREX2 was expressed in HEK293 cells with or without co-expression of FLAG/HA Gβγ and then was purified after being treated with either 500 nm GDC0941, insulin (with co-expression of FLAG/HA Gβγ), or 100 nm calyculin A. The ability of increasing doses of PIP3 in A or Gβγ in B to stimulate PREX2 GEF activity toward GST Rac1 was assessed in an in vitro GEF assay. Data at each PIP3 and Gβγ concentration are means ± S.D. for three experiments, and # represents p < 0.05 for GDC0941 versus Gβγ/insulin, and * represents p < 0.005 for GDC0941 versus calyculin A by t test. C, Western blot analysis of endogenous PREX2 that was immunoprecipitated (IP) from HEK293 cells that were starved and treated with insulin for the indicated times. D, Western blot analysis of V5 PREX2-expressing HEK293 cells that were starved and then treated with insulin for the indicated times. For quantification of phosphorylation in C and D, intensity of the band was normalized to total protein of that sample. The highest value was set to 1, and the remaining samples were normalized to this value. E, Western blot analysis of starved or insulin-treated HEK293 cells expressing V5 PREX2 with or without MYC Rac1 WT or the dominant negative mutant T17N. F, Western blot analysis of starved or insulin-treated HEK293 cells expressing V5 PREX2 WT or the GEF dead E30A/N212A mutant.