FIGURE 6.
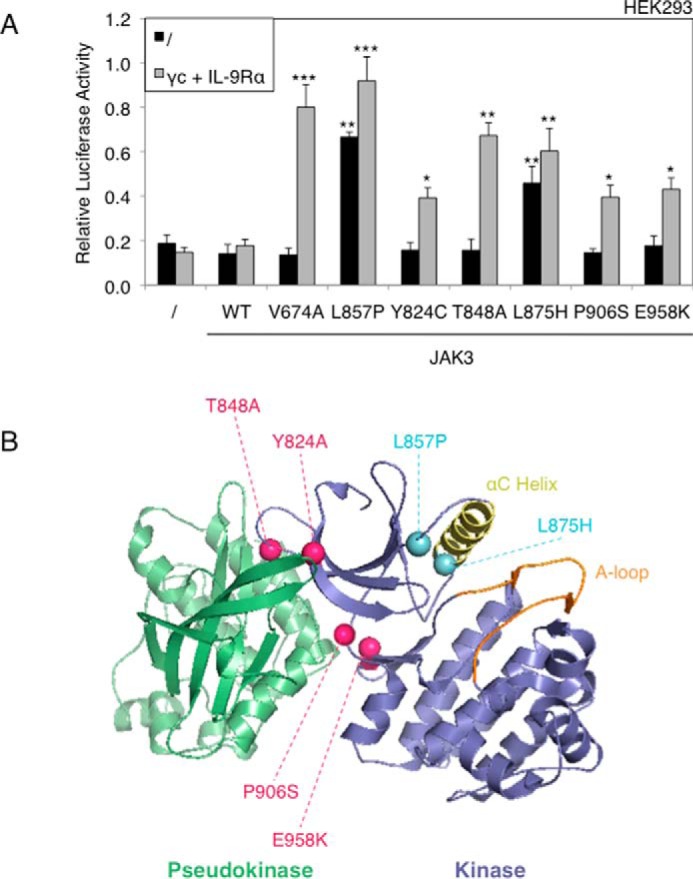
The majority of kinase domain mutants are receptor-dependent, except JAK3L857P and JAK3L875H. A, HEK293 cells were transiently co-transfected either with JAK3WT or different JAK3 mutants (V674A, L857P, Y824A, T848A, L875H, P906S, and E958K), in addition to the STAT5-responsive luciferase reporter pLHRE-luc (firefly luciferase) and the pRLTK plasmid (Renilla luciferase) as transfection control. 24 h post-transfection, the cells were subjected to a luciferase assay. The relative luciferase activity corresponds to the firefly luciferase light emission values divided by the Renilla luciferase light emission values. The results are means ± standard deviation of means of three different experiments, each performed in triplicate. A Kruskal-Wallis test with Dunn correction was performed to determine p values between the control condition without JAK3 and the WT or mutant forms of JAK3 for each condition. *, p < 0.05; **, p < 0.01; ***, p < 0.001. B, localization of ALL-associated JAK3 mutants of the kinase domain. The figure represents the kinase and pseudo-kinase domains of JAK3 based on the recent TYK2 JH1/JH2 crystal structure (33). The kinase domain is shown in indigo, with the αC helix in yellow and the activation loop in orange. The adjacent pseudokinase domain is shown in green. Mutated residues close to the pseudokinase domain are indicated with pink balls, and mutated residues close to the αC helix are indicated with light blue balls.
