FIGURE 1.
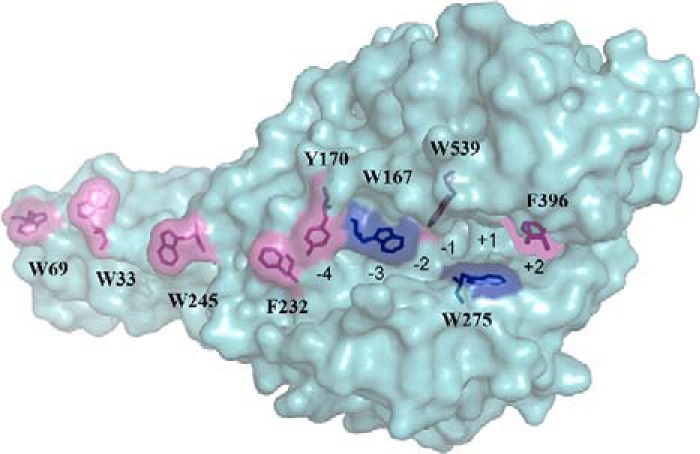
Structure of S. marcescens ChiA. The chitin binding cleft of ChiA is aligned with aromatic residues. The active site rests in the catalytic domain containing four substrate (−4 to −1) and three product (+1 to +3) NAG unit binding sites. The hydrolysis of glycosidic bond takes place between binding sites −1 and +1. The CBM is rigidly connected to the catalytic domain and provides additional NAG unit substrate-binding sites (−13 to −5). Two Trp residues that were replaced with Ala in ChiA variants, ChiA-W167A and ChiA-W275A, studied here are indicated with dark blue color. The rest of the aromatic residues involved in chitin binding are colored pink.
