FIGURE 7.
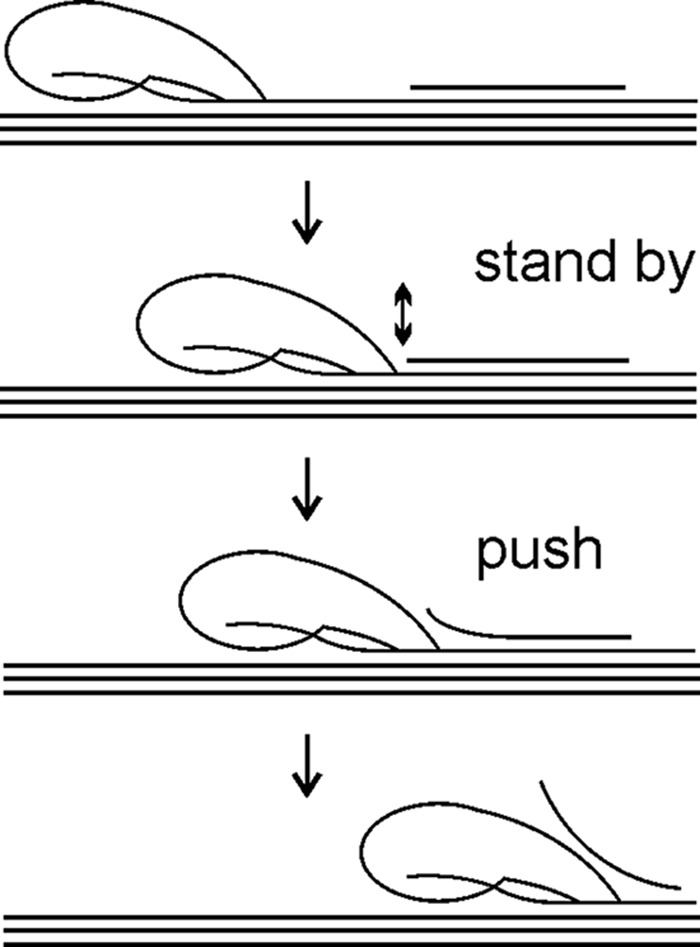
Possible mechanism of degradation of recalcitrant polysaccharide by processive enzyme. An enzyme progresses along polymer chain hydrolyzing it in parallel until it encounters an obstacle (represented by solitary chain on polymer surface). An enzyme halted by the obstacle is bound to the chitin chain with the reducing chain end in the substrate-binding site −1, or +1 if the glycosidic bond is not correctly positioned for hydrolysis. Strong interactions between enzyme and polymer chain in the substrate-binding sites ensure low off-rate and gives to an enzyme a stand by status. At the same time, strong binding of the chain end in the product sites provides the enzyme with pushing potential and forces it to move forward. If obstacle is attached to the polymer surface through non-covalent interactions, a part of it may occasionally “melt” from the polymer surface (represented by two-headed arrow). In such a case, a stand-by enzyme with high pushing potential can move forward and disintegrate the obstacle. Weak binding in substrate-binding sites (like ChiA-W167A) causes the enzyme to dissociate and does not provide sufficient population of stand-by enzymes. Weak binding in product sites (like ChiA-W275A) does not provide the enzyme with strong enough pushing potential to move forward and disintegrate the obstacle. Therefore, an apparent processivity of ChiA-W167A and ChiA-W275A is determined by the average length of obstacle-free bath on polymer. The wild type enzyme can increase the length of the processive runs by pushing away the obstacles.
