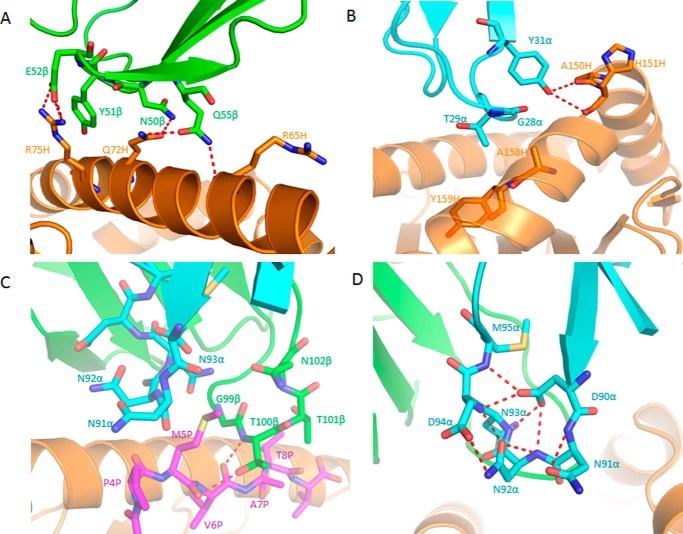
FIGURE 4.
Interactions of TCR C25 with HLA-A2 and the NLV peptide. A, interactions between CDR2β (green) of C25 and the HLA-A2 α1 helix (orange). The side chains of contacting residues are drawn in stick representation with carbon atoms in green (CDR2β) or orange (HLA-A2), nitrogen atoms in blue, and oxygen atoms in red. Hydrogen bonds are indicated by red dashed lines. B, interactions between CDR1α (cyan) of C25 and the HLA-A2 α2 helix (orange). C, interactions between C25 and the NLV peptide (magenta). Peptide residues are identified by a one-letter amino acid designation followed by position (P) number. CDR3α (cyan) and CDR3β (green) form a pocket that accommodates the side chain of P5 Met. The sulfur atom of P5 Met is yellow. D, conformational stabilization of CDR3α of C25 by a dense network of eight intraloop hydrogen bonds.