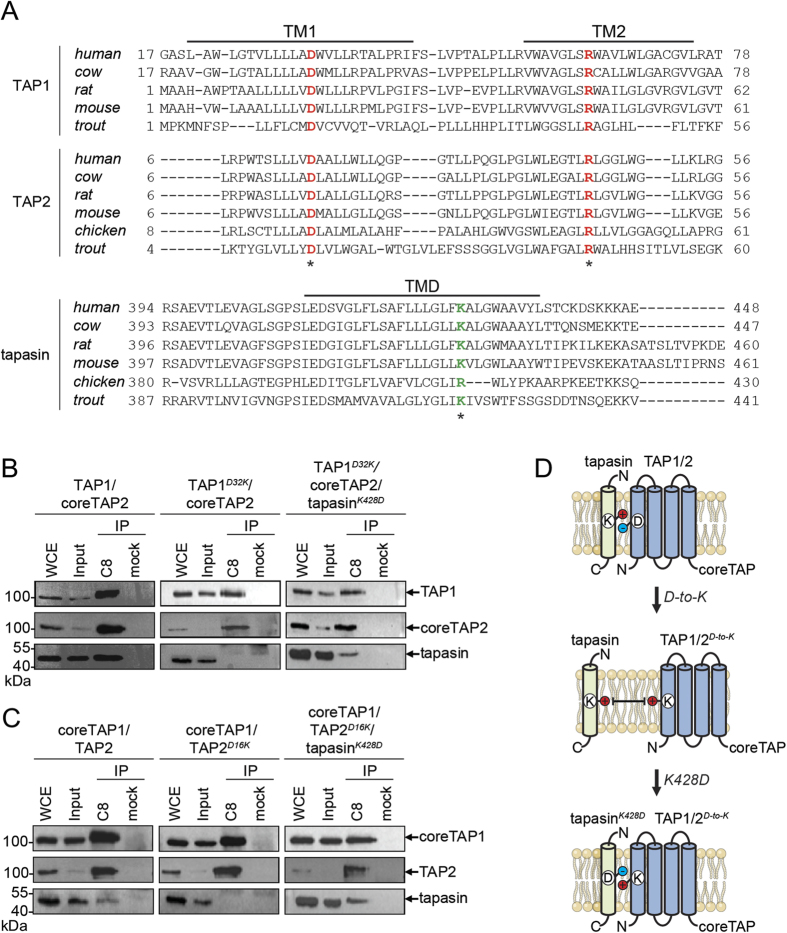
Figure 2. An inter-molecular salt bridge defines the specificity of the TAP-tapasin interaction.
(A) Sequence alignments for TAP1, TAP2, and tapasin of human, cow, rat, mouse, chicken, and trout are shown. Conserved Asp and Arg residues in TAP and the conserved lysine residue in tapasin are marked in red and green, respectively (asterix). Notably, chicken tapasin has an arginine instead of a lysine and chicken TAP1 has no TMD0. (B,C) Interaction of full-length human TAP1D-to-K and TAP2D-to-K with tapasin can be rescued by co-expressing tapasinK428D in HeLa cells (charge exchange). TAP complexes fused to mVenus and mCerulean (ref. 24) were selectively purified via tandem affinity purification (IP), and analyzed by SDS-PAGE followed by immunoblotting. Whole cell extract (WCE) and input represent 1/20 aliquot of the precipitate. (D) Schematic representation of the TAP-tapasin interaction highlighting the specificity that is determined by an inter-subunit salt bridge in the ER membrane. A D-to-K exchange in TAP1/2 interrupts the interaction with tapasin, whereas an additional K-to-D exchange in tapasin leads to a rescue.