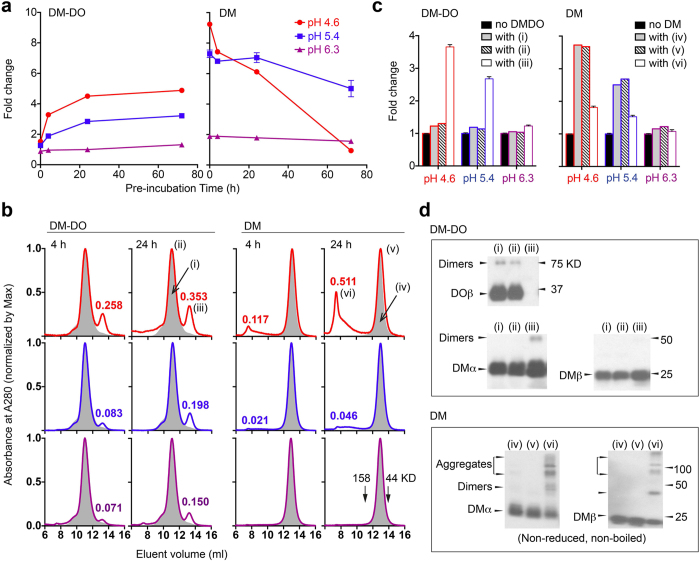
Figure 4. pH-promoted generation of free DM from DM-DO ectodomains.
(a) DM-DOwt or DM (800 nM) was pre-incubated at the indicated pH at 37 °C and then mixed with DR4 (5 nM) and HA306–318 (1 μM). HA loading at the corresponding pH was carried out at 37 °C for 1.5 h. The difference in HA loading with or without acid pre-treated DM-DO (left) or DM (right) was quantified as fold change and plotted against pre-incubation time. (b) DM-DOwt or DM was incubated at pH 4.6 (upper panels), 5.4 (middle panels), or 6.3 (lower panels) for indicated times, and the resultant proteins were separated by size exclusion chromatography. The absorbance against eluent volume was normalized. Gray area (i) and (iv) represents eluents of non-treated DM-DOwt and DM, respectively. Relative absorbance of peaks of both novel eluents (iii) and (vi) observed following pre-incubation is indicated. (c) Non-treated DM-DOw (i) or DM (iv) and resultant proteins (ii) and (iii) or (v) and (vi) from pH 4.6 pre-treated DM-DOwt or DM were collected separately and used at the same concentration (100 nM DM equivalent) in the HA loading assay described in (a). Normalization was performed with peptide loading at the corresponding pH conditions without DM and results are indicated as fold change. (d) Proteins in (i–vi) were reduced and boiled, unless otherwise indicated, before separation by gel electrophoresis and then detected with DO- or DM-specific antibodies by western blot. Dimers may include heterodimers and/or homodimers. Error bars represent SEM for triplicates (a,c). Data are representative of five (a) or three (b–d) experiments.