Figure 2. Site-directed mutagenesis of PSKR1 activation segment or KD residues impairs kinase activity in vitro.
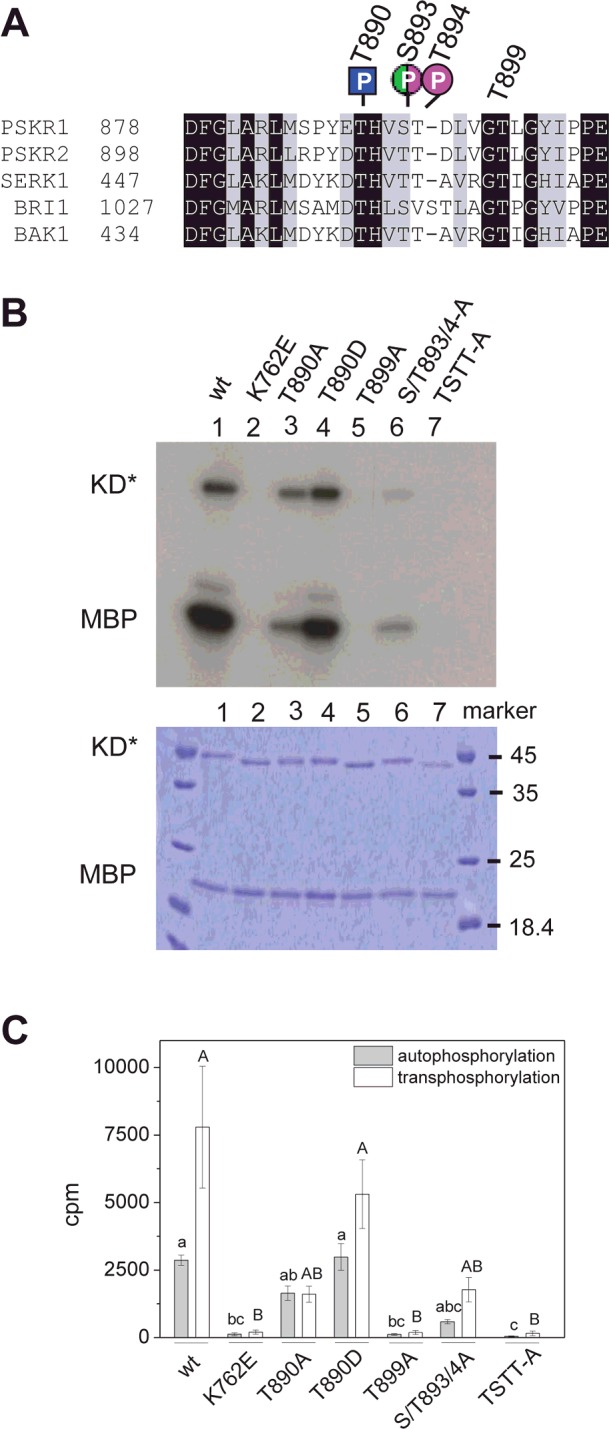
(A) Alignment of the activation segments encompassing the AL (Ser886–Thr894 in PSKR1) of PSKR1, PSKR2, SERK1, BRI1 and BAK1. The phosphorylation sites of PSKR1 that were identified in vitro (magenta), in planta (green) or in the kinase-inactive variant in E. coli (blue) are indicated. (B) Top: autoradiograph of recombinant PSKR1-KD variants (KD*) showing autophosphorylation of KD* and trans-phosphorylation of MBP: wild-type (lane 1), the kinase-inactive His6–PSKR1(K762E)-KD (lane 2), His6–PSKR1(T890A) (lane 3), His6–PSKR1(T890D) (lane 4), His6–PSKR1(T899A) (lane 5), His6–PSKR1(S893A/T894A) (lane 6), and His6–PSKR1(TSTT-A) (lane 7). Bottom: Coomassie Blue-stained gel as a control for protein loading. (C) Mean±S.E.M. radioactivity (n=6 from three independent experiments) of trans-phosphorylated [32P]MBP and autophosphorylated [32P]KD* is given as c.p.m. Kruskal–Wallis all-pairwise comparisons were performed for autophosporylation (lower-case letters) and for trans-phosphorylation (capital letters) activities. TSTT-A, T890A/S893A/T894A/T899A; wt, wild-type.
